Patient-derived cancer organoid tracking with wide-field one-photon redox imaging to assess treatment response
- PMID: 33754540
- PMCID: PMC7983069
- DOI: 10.1117/1.JBO.26.3.036005
Patient-derived cancer organoid tracking with wide-field one-photon redox imaging to assess treatment response
Abstract
Significance: Accessible tools are needed for rapid, non-destructive imaging of patient-derived cancer organoid (PCO) treatment response to accelerate drug discovery and streamline treatment planning for individual patients.
Aim: To segment and track individual PCOs with wide-field one-photon redox imaging to extract morphological and metabolic variables of treatment response.
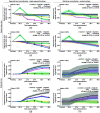
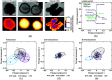
Approach: Redox imaging of the endogenous fluorophores, nicotinamide dinucleotide (NADH), nicotinamide dinucleotide phosphate (NADPH), and flavin adenine dinucleotide (FAD), was used to monitor the metabolic state and morphology of PCOs. Redox imaging was performed on a wide-field one-photon epifluorescence microscope to evaluate drug response in two colorectal PCO lines. An automated image analysis framework was developed to track PCOs across multiple time points over 48 h. Variables quantified for each PCO captured metabolic and morphological response to drug treatment, including the optical redox ratio (ORR) and organoid area.
Results: The ORR (NAD(P)H/(FAD + NAD(P)H)) was independent of PCO morphology pretreatment. Drugs that induced cell death decreased the ORR and growth rate compared to control. Multivariate analysis of redox and morphology variables identified distinct PCO subpopulations. Single-organoid tracking improved sensitivity to drug treatment compared to pooled organoid analysis.
Conclusions: Wide-field one-photon redox imaging can monitor metabolic and morphological changes on a single organoid-level, providing an accessible, non-destructive tool to screen drugs in patient-matched samples.
Keywords: autofluorescence; cancer organoid; drug screening; image analysis; redox imaging; tracking.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

