Increased SARS-CoV-2 Testing Capacity with Pooled Saliva Samples
- PMID: 33755009
- PMCID: PMC8007323
- DOI: 10.3201/eid2704.204200
Increased SARS-CoV-2 Testing Capacity with Pooled Saliva Samples
Abstract
We analyzed feasibility of pooling saliva samples for severe acute respiratory syndrome coronavirus 2 testing and found that sensitivity decreased according to pool size: 5 samples/pool, 7.4% reduction; 10 samples/pool, 11.1%; and 20 samples/pool, 14.8%. When virus prevalence is >2.6%, pools of 5 require fewer tests; when <0.6%, pools of 20 support screening strategies.
Keywords: 2019 novel coronavirus disease; COVID-19; SARS-CoV-2; coronavirus disease; diagnostics; respiratory infections; saliva; screening; severe acute respiratory syndrome coronavirus 2; viruses; zoonoses.
Figures
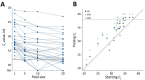
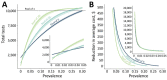
Update of
-
Pooling saliva to increase SARS-CoV-2 testing capacity.medRxiv [Preprint]. 2020 Sep 3:2020.09.02.20183830. doi: 10.1101/2020.09.02.20183830. medRxiv. 2020. Update in: Emerg Infect Dis. 2021 Apr;27(4). doi: 10.3201/eid2704.204200. PMID: 32909003 Free PMC article. Updated. Preprint.
Similar articles
-
Stability of SARS-CoV-2 RNA in Nonsupplemented Saliva.Emerg Infect Dis. 2021 Apr;27(4):1146-1150. doi: 10.3201/eid2704.204199. Emerg Infect Dis. 2021. PMID: 33754989 Free PMC article.
-
Saliva sample pooling for the detection of SARS-CoV-2.J Med Virol. 2021 Mar;93(3):1506-1511. doi: 10.1002/jmv.26460. Epub 2020 Sep 29. J Med Virol. 2021. PMID: 32841429 Free PMC article.
-
Assessment of the Effective Sensitivity of SARS-CoV-2 Sample Pooling Based on a Large-Scale Screening Experience: Retrospective Analysis.JMIR Public Health Surveill. 2024 Sep 24;10:e54503. doi: 10.2196/54503. JMIR Public Health Surveill. 2024. PMID: 39316785 Free PMC article.
-
Diagnosis of SARS-CoV-2 by RT-PCR Using Different Sample Sources: Review of the Literature.Ear Nose Throat J. 2021 Apr;100(2_suppl):131S-138S. doi: 10.1177/0145561320953231. Epub 2020 Aug 31. Ear Nose Throat J. 2021. PMID: 32865458 Free PMC article. Review.
-
Pooled Testing Strategies for SARS-CoV-2 diagnosis: A comprehensive review.Diagn Microbiol Infect Dis. 2021 Oct;101(2):115432. doi: 10.1016/j.diagmicrobio.2021.115432. Epub 2021 May 17. Diagn Microbiol Infect Dis. 2021. PMID: 34175613 Free PMC article. Review.
Cited by
-
Pooled Saliva Specimens for SARS-CoV-2 Testing.medRxiv [Preprint]. 2020 Oct 5:2020.10.02.20204859. doi: 10.1101/2020.10.02.20204859. medRxiv. 2020. Update in: J Clin Microbiol. 2021 Feb 18;59(3):e02486-20. doi: 10.1128/JCM.02486-20. PMID: 33052363 Free PMC article. Updated. Preprint.
-
Diagnostic Approaches for COVID-19 and Its Associated Complications.Diagnostics (Basel). 2021 Nov 9;11(11):2071. doi: 10.3390/diagnostics11112071. Diagnostics (Basel). 2021. PMID: 34829418 Free PMC article. Review.
-
Safe and effective pool testing for SARS-CoV-2 detection.J Clin Virol. 2021 Dec;145:105018. doi: 10.1016/j.jcv.2021.105018. Epub 2021 Oct 28. J Clin Virol. 2021. PMID: 34775143 Free PMC article.
-
Increasing SARS-CoV-2 RT-qPCR testing capacity by sample pooling.Int J Infect Dis. 2021 Feb;103:19-22. doi: 10.1016/j.ijid.2020.11.155. Epub 2020 Nov 19. Int J Infect Dis. 2021. PMID: 33220439 Free PMC article.
-
Wide Application of Minimally Processed Saliva on Multiple RT-qPCR Kits for SARS-CoV-2 Detection in Indonesia.Front Cell Infect Microbiol. 2021 Aug 18;11:691538. doi: 10.3389/fcimb.2021.691538. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34485174 Free PMC article.
References
-
- Vogels CBF, Brackney D, Wang J, Kalinich CC, Ott I, Kudo E, et al. SalivaDirect: simple and sensitive molecular diagnostic test for SARS-CoV-2 surveillance [cited 2021 Jan 27]. https://www.cell.com/med/pdf/S2666-6340(20)30076-3.pdf
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

