Increased SARS-CoV-2 Testing Capacity with Pooled Saliva Samples
- PMID: 33755009
- PMCID: PMC8007323
- DOI: 10.3201/eid2704.204200
Increased SARS-CoV-2 Testing Capacity with Pooled Saliva Samples
Abstract
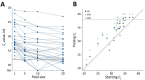
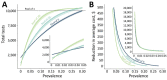
We analyzed feasibility of pooling saliva samples for severe acute respiratory syndrome coronavirus 2 testing and found that sensitivity decreased according to pool size: 5 samples/pool, 7.4% reduction; 10 samples/pool, 11.1%; and 20 samples/pool, 14.8%. When virus prevalence is >2.6%, pools of 5 require fewer tests; when <0.6%, pools of 20 support screening strategies.
Keywords: 2019 novel coronavirus disease; COVID-19; SARS-CoV-2; coronavirus disease; diagnostics; respiratory infections; saliva; screening; severe acute respiratory syndrome coronavirus 2; viruses; zoonoses.
Figures
Update of
-
Pooling saliva to increase SARS-CoV-2 testing capacity.medRxiv [Preprint]. 2020 Sep 3:2020.09.02.20183830. doi: 10.1101/2020.09.02.20183830. medRxiv. 2020. Update in: Emerg Infect Dis. 2021 Apr;27(4). doi: 10.3201/eid2704.204200. PMID: 32909003 Free PMC article. Updated. Preprint.
References
-
- Vogels CBF, Brackney D, Wang J, Kalinich CC, Ott I, Kudo E, et al. SalivaDirect: simple and sensitive molecular diagnostic test for SARS-CoV-2 surveillance [cited 2021 Jan 27]. https://www.cell.com/med/pdf/S2666-6340(20)30076-3.pdf
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

