Effects of domestication on the gut microbiota parallel those of human industrialization
- PMID: 33755015
- PMCID: PMC7987347
- DOI: 10.7554/eLife.60197
Effects of domestication on the gut microbiota parallel those of human industrialization
Abstract
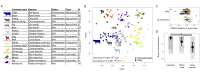
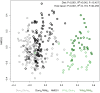
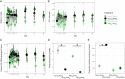
Domesticated animals experienced profound changes in diet, environment, and social interactions that likely shaped their gut microbiota and were potentially analogous to ecological changes experienced by humans during industrialization. Comparing the gut microbiota of wild and domesticated mammals plus chimpanzees and humans, we found a strong signal of domestication in overall gut microbial community composition and similar changes in composition with domestication and industrialization. Reciprocal diet switches within mouse and canid dyads demonstrated the critical role of diet in shaping the domesticated gut microbiota. Notably, we succeeded in recovering wild-like microbiota in domesticated mice through experimental colonization. Although fundamentally different processes, we conclude that domestication and industrialization have impacted the gut microbiota in related ways, likely through shared ecological change. Our findings highlight the utility, and limitations, of domesticated animal models for human research and the importance of studying wild animals and non-industrialized humans for interrogating signals of host-microbial coevolution.
Keywords: canid; domestication; ecology; evolutionary biology; gut microbiota; human; industrialization; mouse; rat.
Plain language summary
Living inside our gastrointestinal tracts is a large and diverse community of bacteria called the gut microbiota that plays an active role in basic body processes like metabolism and immunity. Much of our current understanding of the gut microbiota has come from laboratory animals like mice, which have very different gut bacteria to mice living in the wild. However, it was unclear whether this difference in microbes was due to domestication, and if it could also be seen in other domesticated-wild pairs, like pigs and wild boars or dogs and wolves. A few existing studies have compared the gut bacteria of two species in a domesticated-wild pair. But, studies of isolated pairs cannot distinguish which factors are responsible for altering the microbiota of domesticated animals. To overcome this barrier, Reese et al. sequenced microbial DNA taken from fecal samples of 18 species of wild and related domesticated mammals. The results showed that while domesticated animals have different sets of bacteria in their guts, leaving the wild has changed the gut microbiota of these diverse animals in similar ways. To explore what causes these shared patterns, Reese et al. swapped the diets of two domesticated-wild pairs: laboratory and wild mice, and dogs and wolves. They found this change in diet shifted the gut bacteria of the domesticated species to be more similar to that of their wild counterparts, and vice versa. This suggests that altered eating habits helped drive the changes domestication has had on the gut microbiota. To find out whether these differences also occur in humans, Reese et al. compared the gut microbes of chimpanzees with the microbiota of people living in different environments. The gut microbial communities of individuals from industrialized populations had more in common with those of domesticated animals than did the microbes found in chimpanzees or humans from non-industrialized populations. This suggests that industrialization and domestication have had similar effects on the gut microbiota, likely due to similar kinds of environmental change. Domesticated animals are critical for the economy and health, and understanding the central role gut microbes play in their biology could help improve their well-being. Given the parallels between domestication and industrialization, knowledge gained from animal pairs could also shed light on the human gut microbiota. In the future, these insights could help identify new ways to alter the gut microbiota to improve animal or human health.
© 2021, Reese et al.
Conflict of interest statement
AR, KC, CD, LS, MB, PC, RR, ME, RC No competing interests declared
Figures


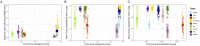





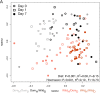
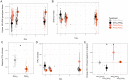


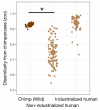
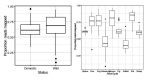
Comment in
-
Taming the beasts inside.Elife. 2021 Mar 23;10:e67634. doi: 10.7554/eLife.67634. Elife. 2021. PMID: 33755018 Free PMC article.
References
-
- Alessandri G, Milani C, Mancabelli L, Mangifesta M, Lugli GA, Viappiani A, Duranti S, Turroni F, Ossiprandi MC, van Sinderen D, Ventura M. Metagenomic dissection of the canine gut microbiota: insights into taxonomic, metabolic and nutritional features. Environmental Microbiology. 2019;21:1331–1343. doi: 10.1111/1462-2920.14540. - DOI - PubMed
-
- Amato KR, Mallott EK, McDonald D, Dominy NJ, Goldberg T, Lambert JE, Swedell L, Metcalf JL, Gomez A, Britton GAO, Stumpf RM, Leigh SR, Knight R. Convergence of human and old world monkey gut microbiomes demonstrates the importance of human ecology over phylogeny. Genome Biology. 2019;20:201. doi: 10.1186/s13059-019-1807-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

