Xpert MTB/RIF and Xpert Ultra assays for screening for pulmonary tuberculosis and rifampicin resistance in adults, irrespective of signs or symptoms
- PMID: 33755189
- PMCID: PMC8437892
- DOI: 10.1002/14651858.CD013694.pub2
Xpert MTB/RIF and Xpert Ultra assays for screening for pulmonary tuberculosis and rifampicin resistance in adults, irrespective of signs or symptoms
Abstract
Background: Tuberculosis is a leading cause of infectious disease-related death and is one of the top 10 causes of death worldwide. The World Health Organization (WHO) recommends the use of specific rapid molecular tests, including Xpert MTB/RIF or Xpert Ultra, as initial diagnostic tests for the detection of tuberculosis and rifampicin resistance in people with signs and symptoms of tuberculosis. However, the WHO estimates that nearly one-third of all active tuberculosis cases go undiagnosed and unreported. We were interested in whether a single test, Xpert MTB/RIF or Xpert Ultra, could be useful as a screening test to close this diagnostic gap and improve tuberculosis case detection.
Objectives: To estimate the accuracy of Xpert MTB/RIF and Xpert Ultra for screening for pulmonary tuberculosis in adults, irrespective of signs or symptoms of pulmonary tuberculosis in high-risk groups and in the general population. Screening "irrespective of signs or symptoms" refers to screening of people who have not been assessed for the presence of tuberculosis symptoms (e.g. cough). To estimate the accuracy of Xpert MTB/RIF and Xpert Ultra for detecting rifampicin resistance in adults screened for tuberculosis, irrespective of signs and symptoms of pulmonary tuberculosis in high-risk groups and in the general population.
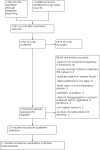
Search methods: We searched 12 databases including the Cochrane Infectious Diseases Group Specialized Register, MEDLINE and Embase, on 19 March 2020 without language restrictions. We also reviewed reference lists of included articles and related Cochrane Reviews, and contacted researchers in the field to identify additional studies.
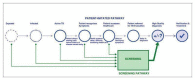
Selection criteria: Cross-sectional and cohort studies in which adults (15 years and older) in high-risk groups (e.g. people living with HIV, household contacts of people with tuberculosis) or in the general population were screened for pulmonary tuberculosis using Xpert MTB/RIF or Xpert Ultra. For tuberculosis detection, the reference standard was culture. For rifampicin resistance detection, the reference standards were culture-based drug susceptibility testing and line probe assays.
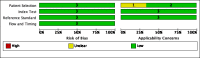
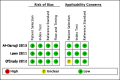
Data collection and analysis: Two review authors independently extracted data using a standardized form and assessed risk of bias and applicability using QUADAS-2. We used a bivariate random-effects model to estimate pooled sensitivity and specificity with 95% credible intervals (CrIs) separately for tuberculosis detection and rifampicin resistance detection. We estimated all models using a Bayesian approach. For tuberculosis detection, we first estimated screening accuracy in distinct high-risk groups, including people living with HIV, household contacts, people residing in prisons, and miners, and then in several high-risk groups combined.
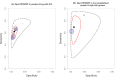
Main results: We included a total of 21 studies: 18 studies (13,114 participants) evaluated Xpert MTB/RIF as a screening test for pulmonary tuberculosis and one study (571 participants) evaluated both Xpert MTB/RIF and Xpert Ultra. Three studies (159 participants) evaluated Xpert MTB/RIF for rifampicin resistance. Fifteen studies (75%) were conducted in high tuberculosis burden and 16 (80%) in high TB/HIV-burden countries. We judged most studies to have low risk of bias in all four QUADAS-2 domains and low concern for applicability. Xpert MTB/RIF and Xpert Ultra as screening tests for pulmonary tuberculosis In people living with HIV (12 studies), Xpert MTB/RIF pooled sensitivity and specificity (95% CrI) were 61.8% (53.6 to 69.9) (602 participants; moderate-certainty evidence) and 98.8% (98.0 to 99.4) (4173 participants; high-certainty evidence). Of 1000 people where 50 have tuberculosis on culture, 40 would be Xpert MTB/RIF-positive; of these, 9 (22%) would not have tuberculosis (false-positives); and 960 would be Xpert MTB/RIF-negative; of these, 19 (2%) would have tuberculosis (false-negatives). In people living with HIV (1 study), Xpert Ultra sensitivity and specificity (95% CI) were 69% (57 to 80) (68 participants; very low-certainty evidence) and 98% (97 to 99) (503 participants; moderate-certainty evidence). Of 1000 people where 50 have tuberculosis on culture, 53 would be Xpert Ultra-positive; of these, 19 (36%) would not have tuberculosis (false-positives); and 947 would be Xpert Ultra-negative; of these, 16 (2%) would have tuberculosis (false-negatives). In non-hospitalized people in high-risk groups (5 studies), Xpert MTB/RIF pooled sensitivity and specificity were 69.4% (47.7 to 86.2) (337 participants, low-certainty evidence) and 98.8% (97.2 to 99.5) (8619 participants, moderate-certainty evidence). Of 1000 people where 10 have tuberculosis on culture, 19 would be Xpert MTB/RIF-positive; of these, 12 (63%) would not have tuberculosis (false-positives); and 981 would be Xpert MTB/RIF-negative; of these, 3 (0%) would have tuberculosis (false-negatives). We did not identify any studies using Xpert MTB/RIF or Xpert Ultra for screening in the general population. Xpert MTB/RIF as a screening test for rifampicin resistance Xpert MTB/RIF sensitivity was 81% and 100% (2 studies, 20 participants; very low-certainty evidence), and specificity was 94% to 100%, (3 studies, 139 participants; moderate-certainty evidence).
Authors' conclusions: Of the high-risks groups evaluated, Xpert MTB/RIF applied as a screening test was accurate for tuberculosis in high tuberculosis burden settings. Sensitivity and specificity were similar in people living with HIV and non-hospitalized people in high-risk groups. In people living with HIV, Xpert Ultra sensitivity was slightly higher than that of Xpert MTB/RIF and specificity similar. As there was only one study of Xpert Ultra in this analysis, results should be interpreted with caution. There were no studies that evaluated the tests in people with diabetes mellitus and other groups considered at high-risk for tuberculosis, or in the general population.
Copyright © 2021 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
AES received funding from USAID, administered by the World Health Organization Global TB Programme, Switzerland. She has received salary compensation from the University of Washington, where she is an Acting Assistant Professor in Global Health and Medicine/Infectious Diseases. A portion of her salary derives from NIH grants and from grants from the Bill & Melinda Gates Foundation.
JMR received funding from USAID, administered by the World Health Organization Global TB Programme, Switzerland. JMR has grants/grants pending to her host institution from US National Institutes of Health, KNCV TB Foundation, and The Global Fund to Fight AIDS, TB, and Malaria, The Firland Foundation.
MY has no known conflicts of interest to declare.
IS has no known conflicts of interest to declare.
MK has received funding from USAID, administered by the World Health Organization Global TB Programme, Switzerland for related systematic reviews.
ND has no known conflicts of interest to declare.
KRS has received financial support from Cochrane Infectious Diseases, UK, McGill University, Canada, and USAID, USA, administered by the World Health Organization (WHO) Global TB Programme, Switzerland, for the preparation of systematic reviews and educational materials, consultancy fees from Foundation for Innovative New Diagnostics (FIND), Switzerland (for the preparation of systematic reviews and GRADE tables), honoraria, and travel support to attend WHO guideline meetings.
DJH has received funding from USAID, administered by the World Health Organization Global TB Programme, Switzerland for related systematic reviews.
Figures




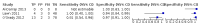





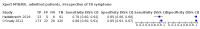


Update of
References
References to studies included in this review
Al‐Darraji 2013 {published data only}
Al‐Darraji 2016 {published and unpublished data}
-
- Al-Darraji HA, Altice FL, Kamarulzaman A. Undiagnosed pulmonary tuberculosis among prisoners in Malaysia: an overlooked risk for tuberculosis in the community. Tropical Medicine and International Health 2016;21(8):1049-58. - PubMed
Balcha 2014 {published data only}
Beyanga 2018 {published data only}
Bjerrum 2016 {published data only}
-
- Bjerrum S, Oliver-Commey J, Kenu E, Lartey M, Newman MJ, Addo KK, et al. Tuberculosis and non-tuberculous mycobacteria among HIV-infected individuals in Ghana. Tropical Medicine & International Health 2016;21(9):1181-90. - PubMed
Dorman 2012 {published data only}
Heidebrecht 2016 {published data only}
Henostroza 2016 {published data only}
-
- Henostroza G, Harris JB, Chitambi R, Siyambango M, Turnbull ER, Maggard KR, et al. High prevalence of tuberculosis in newly enrolled HIV patients in Zambia: need for enhanced screening approach. International Journal of Tuberculosis and Lung Disease 2016;20(8):1033-9. - PubMed
Kempker 2019 {published data only}
LaCourse 2016 {published data only}
-
- LaCourse SM, Cranmer LM, Matemo D, Kinuthia J, Richardson BA, John-Stewart G, et al. Tuberculosis case finding in HIV-Infected pregnant women in Kenya reveals poor performance of symptom screening and rapid diagnostic tests. Journal of the Acquired Immune Deficiency Syndrome 2016;71(2):219-27. - PMC - PubMed
Lawn 2011 {published data only}
Lawn 2012 {published data only}
Lopez‐Varela 2019 {published data only}
-
- López-Varela E, Respeito D, Blanco S, Gimo M, Sacoor C, Naniche D, et al. High yield of home-based TB diagnosis among newly diagnosed patients with HIV. Journal of the Acquired Immune Deficiency Syndrome 2019;80(4):e103-e105. - PubMed
Mollel 2017 {published data only}
-
- Mollel EW, Chilongola JO, Mpagama SG, Kibiki GS. Evaluation of Xpert MTB/Rif performance for diagnosis of tuberculosis among HIV positive patients in northern Tanzania. Tanzania Journal of Health Research 2017;19(1):1-9.
Ntinginya 2012 {published data only}
-
- Ntinginya EN, Squire SB, Millington KA, Mtafya B, Saathoff E, Heinrich N, et al. Performance of the Xpert® MTB/RIF assay in an active case-finding strategy: a pilot study from Tanzania. International Journal of Tuberculosis and Lung Disease 2012;16(11):1468-70. - PubMed
O'Grady 2012 {published data only}
-
- O'Grady J, Bates M, Chilukutu L, Mzyece J, Cheelo B, Chilufya M, et al. Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting where tuberculosis and HIV infection are highly endemic. Clinical Infectious Diseases 2012;55(9):1171-8. - PubMed
Reeve 2019a {published and unpublished data}
-
- Reeve B, Ndlangalavu G, Palmer Z, Jackson J, Dolby T, Helden P, et al. Accuracy of Xpert Ultra and Xpert MTB/RIF in people living with HIV initiating antiretroviral treatment who have minimal TB symptoms. International Journal of Tuberculosis and Lung Diseases 2019;3(10):S115.
Reeve 2019b {published and unpublished data}
-
- Reeve B, Ndlangalavu G, Palmer Z, Jackson J, Dolby T, Helden P, et al. Accuracy of Xpert Ultra and Xpert MTB/RIF in people living with HIV initiating antiretroviral treatment who have minimal TB symptoms. International Journal of Tuberculosis and Lung Diseases 2019;3(10):S115.
Santos 2020 {published and unpublished data}
Tahseen 2018 {published data only}
-
- Tahseen S, Shahnawaz H, Riaz U, Khanzada FM, Hussain A, Aslam W, et al. Systematic case finding for tuberculosis in HIV-infected people who inject drugs: experience from Pakistan. International Journal of Tuberculosis and Lung Disease 2018;22(2):187-193. - PubMed
References to studies excluded from this review
Adams 2015 {published data only}
-
- Adams CD, Velez JD, Martinez LF. Xpert((R)) MTB/RIF and very low positive detection in bronchoalveolar lavage: diagnostic concerns. International Journal of Tuberculosis and Lung Disease 2015;19(7):871-3. - PubMed
Adejumo 2018 {published data only}
Adetunji 2019 {published data only}
-
- Adetunji SO, Donbraye E, Ekong MJ, Adetunji BI. Rifampicin-resistant tuberculosis among known HIV-infected patients in Oyo State, Nigeria. Journal of Immunoassay and Immunochemistry 2019;40(3):289-99. - PubMed
Agizew 2017 {published data only}
Aia 2016 {published data only}
Antonenka 2013 {published data only}
Ardizzoni 2015 {published data only}
Ardizzoni 2020 {published data only}
Assefa 2019 {published data only}
Auld 2016a {published data only}
-
- Auld AF, Agizew T, Pals S, Finlay A, Ndwapi N, Boyd R, et al. Implementation of a pragmatic, stepped-wedge cluster randomized trial to evaluate impact of Botswana's Xpert MTB/RIF diagnostic algorithm on TB diagnostic sensitivity and early antiretroviral therapy mortality. BMC Infectious Diseases 2016;16(1):606. - PMC - PubMed
Auld 2016b {published data only}
Auld 2020 {published data only}
Awan 2018 {published data only}
-
- Awan WM, Zaidi SM, Habib SS, Khowaja S, Malik A, Khan U, et al. Impact of scaling up Xpert((R)) MTB/RIF testing for the detection of rifampicin-resistant TB cases in Karachi, Pakistan. International Journal of Tuberculosis and Lung Disease 2018;22(8):899-904. - PubMed
Ayala 2016 {published data only}
-
- Ayala G, Garay J, Aragon M, Decroo T, Zachariah R. Trends in tuberculosis notification and treatment outcomes in prisons: a country-wide assessment in El Salvador from 2009-2014. Pan American Journal of Public Health (Revista Panamericana de Salud Pública) 2016;39(1):38-43. - PubMed
Bablishvili 2015 {published data only}
Bacells 2016 {published data only}
-
- Bacells ME, Huilcaman M, Pena C, Castillo C, Carvajal C, Scioscia N, et al. M-tuberculosis DNA detection in nasopharyngeal mucosa can precede tuberculosis development in contacts. International Journal of Tuberculosis and Lung Disease 2016;20(6):848-52. - PubMed
Balcha 2014a {published data only}
-
- Balcha TT, Winqvist N, Sturegard E, Skogmar S, Reepalu A, Jemal ZH, et al. Detection of lipoarabinomannan in urine for identification of active tuberculosis among HIV-positive adults in Ethiopian health centres. Tropical Medicine & International Health 2014;19(6):734-42. - PubMed
Balcha 2015 {published data only}
Basir 2019 {published data only}
Bassett 2019 {published data only}
Benjamin 2019 {published data only}
Bhardwaj 2019 {published data only}
-
- Bhardwaj A, Khan S, Kumar A, George L, Mehta A, Radhakrishnan K. Assessing the utility of GeneXpert MTB/Rif Assay in a tertiary care centre in Southern India with established microscopy and liquid culture facilities. Journal of the Association of Physicians of India 2019;67(8):31-4. - PubMed
Bjerrum 2015 {published data only}
Blakemore 2011 {published data only}
Boum 2016 {published data only}
Byashalira 2019 {published data only}
-
- Byashalira K, Mbelele P, Semvua H, Chilongola J, Semvua S, Liyoyo A, et al. Clinical outcomes of new algorithm for diagnosis and treatment of tuberculosis sepsis in HIV patients. International Journal of Mycobacteriology 2019;8(4):313-9. - PubMed
Calligaro 2017 {published data only}
-
- Calligaro GL, Zijenah LS, Peter JG, Theron G, Buser V, McNerney R, et al. Effect of new tuberculosis diagnostic technologies on community-based intensified case finding: a multicentre randomised controlled trial. Lancet Infectious Diseases 2017;17(4):441-50. - PubMed
Carmone 2017 {published data only}
Cavanaugh 2016 {published data only}
Celik 2015 {published data only}
-
- Celik C, Gozel MG, Bakici MZ, Berk S, Ozsahin SL, Gulturk E. Applicability of Xpert MTB/RIF assay for routine diagnosis of tuberculosis: a four-year single-center experience. Turkish Journal of Medical Sciences 2015;45(6):1329-34. - PubMed
Charoensook 2018 {published data only}
-
- Charoensook P, Upala P, Anuwatnonthakate A, Ruanjai T, Apidechkul T. Pulmonary tuberculosis screening and quality of life among migrant workers, Northern Thailand. Journal of Infection in Developing Countries 2018;12(12):1052-61. - PubMed
Chry 2020 {published data only}
Chumpa 2020 {published data only}
-
- Chumpa N, Kawkitinarong K, Rotcheewaphan S, Sawatpanich A, Petsong S, Tumwasorn S, et al. Evaluation of AnyplexTM II MTB/MDR kit's performance to rapidly detect isoniazid and rifampicin resistant Mycobacterium tuberculosis from various clinical specimens. Molecular Biology Reports 2020;47(4):2501-8. - PubMed
Deshmukh 2020 {published data only}
-
- Deshmukh S, Atre S, Chavan A, Raskar S, Sawant T, Mave V, et al. Assessment of the Xpert assay among adult pulmonary tuberculosis suspects with and without diabetes mellitus. International Union against Tuberculosis and Lung Disease 2020;24(1):113-7. - PubMed
Ekeke 2020 {published data only}
-
- Ekeke N, Aniwada E, Chukwu J, Nwafor C, Meka A, Chukwuka A, et al. Screening diabetes mellitus patients for tuberculosis in Southern Nigeria: a pilot study. Advances in Respiratory Medicine 2020;88(1):6-12. - PubMed
Farra 2017 {published data only}
-
- Farra A, Manirakiza A, Yambiyo BM, Zandanga G, Lokoti B, Berlioz-Arthaud A, et al. Surveillance of rifampicin resistance with GeneXpert MTB/RIF in the National Reference Laboratory for tuberculosis at the Institut Pasteur in Bangui, 2015-2017. Open Forum Infectious Diseases 2019;6(3):ofz075. - PMC - PubMed
Floridia 2017 {published data only}
-
- Floridia M, Ciccacci F, Andreotti M, Hassane A, Sidumo Z, Magid NA, et al. Tuberculosis case finding with combined rapid point-of-care assays (Xpert MTB/RIF and Determine TB LAM) in HIV-positive individuals starting antiretroviral therapy in Mozambique. Clinical Infectious Diseases 2017;65(11):1878-83. - PubMed
Gautam 2019 {published data only}
-
- Gautam H, Singla M, Jain R, Lodha R, Kabra SK, Singh UB. Point-of-care urine lipoarabinomannan antigen detection for diagnosis of tuberculosis in children. International Journal of Tuberculosis and Lung Disease 2019;23(6):714-9. - PubMed
Gelalcha 2017 {published data only}
Gizachew 2017 {published data only}
Gupta‐Wright 2018 {published data only}
-
- Gupta-Wright A, Corbett EL, Oosterhout JJ, Wilson D, Grint D, Alufandika-Moyo M, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (STAMP): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018;392(10144):292-301. - PMC - PubMed
Gursoy 2016 {published data only}
-
- Gursoy NC, Yakupogullari Y, Tekerekoglu MS, Otlu B. [Evaluation of the diagnostic performance of Xpert MTB/RIF test for the detection of Mycobacterium tuberculosis and rifampin resistance in clinical samples]. Mikrobiyoloji Bulteni 2016;50(2):196-204. - PubMed
Habeenzu 2017 {published data only}
-
- Habeenzu C, Nakajima C, Solo E, Bwalya P, Kajino K, Miller M, et al. Evaluation of in-house loop-mediated isothermal amplification for tuberculosis diagnosis compared with Xpert MTB/RIF. Journal of Infection in Developing Countries 2017;11(6):440-4. - PubMed
Habte 2016 {published data only}
-
- Habte D, Melese M, Hiruy N, Gashu Z, Jerene D, Moges F, et al. The additional yield of GeneXpert MTB/RIF test in the diagnosis of pulmonary tuberculosis among household contacts of smear positive TB cases. International Journal of Infectious Diseases 2016;49:179-84. - PubMed
Hanifa 2016 {published data only}
Head 2019 {published data only}
-
- Head IM, Lazarus R. Use of Xpert MTB/RIF in a low prevalence setting in the Southwest of England. Journal of Infection 2019;S0163-4453(19):30345-7. - PubMed
Hiruy 2018 {published data only}
-
- Hiruy N, Melese M, Habte D, Jerene D, Gashu Z, Alem G, et al. Comparison of the yield of tuberculosis among contacts of multidrug-resistant and drug-sensitive tuberculosis patients in Ethiopia using GeneXpert as a primary diagnostic test. International Journal of Infectious Diseases 2018;71:4-8. - PubMed
Ho 2016 {published data only}
-
- Ho J, Nguyen PT, Nguyen TA, Tran KH, Van Nguyen S, Nguyen NV, et al. Reassessment of the positive predictive value and specificity of Xpert MTB/RIF: a diagnostic accuracy study in the context of community-wide screening for tuberculosis. Lancet Infectious Diseases 2016;16(9):1045-51. - PubMed
Hosseinipour 2016 {published data only}
-
- Hosseinipour MC, Bisson GP, Miyahara S, Sun X, Moses A, Riviere C, et al. Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet 2016;387(10024):1198-209. - PMC - PubMed
Huang 2018 {published data only}
-
- Huang H, Zhang Y, Li S, Wang J, Chen J, Pan Z, et al. Rifampicin resistance and multidrug-resistant tuberculosis detection using Xpert MTB/RIF in Wuhan, China: a retrospective study. Microbial Drug Resistance 2018;24(5):675-9. - PubMed
Huerga 2020 {published data only}
-
- Huerga H, Cossa L, Manhiça I, Bastard M, Telnov A, Molfino L, et al. Systematic, point-of-care urine lipoarabinomannan (Alere TB-LAM) assay for diagnosing tuberculosis in severely immunocompromised HIV-Positive ambulatory patients. American Journal of Tropical Medicine and Hygiene 2020;102(3):562-6. - PMC - PubMed
Huh 2019 {published data only}
Kamenska 2019 {published data only}
-
- Kamenska N, Nabirova D, Davtyan K, Davtyan H, Zachariah R, Aslanyan G. Strategies for active detection of tuberculosis in Ukraine: Comparative effectiveness amongst key populations (2014-2018). Journal of Infection in Developing Countries 2019;13(7.1):89s-94s. - PubMed
Kerkhoff 2014 {published data only}
Kurbaniyazova 2017 {published data only}
-
- Kurbaniyazova G, Joncevska M, Kalon S, Kalmambetova G, Mohr T, Toktogonova A, et al. Results of Xpert MTB/RIF implementation in Kyrgyzstan. International Journal of Tuberculosis and Lung Disease 2017;21(3):333-7. - PubMed
Kuyinu 2018 {published data only}
-
- Kuyinu YA, Odugbemi BA, Salisu-Olatunji SO, Adepoju FO, Odusanya OO. Characteristics of Mycobacterium tuberculosis positive patients screened for drug-resistant tuberculosis at a tertiary health facility in Lagos, Nigeria. Journal of the National Medical Association 2018;110(1):88-91. - PubMed
LaCourse 2014 {published data only}
LaCourse 2018 {published data only}
Lawn 2012a {published data only}
Lawn 2012b {published data only}
-
- Lawn SD, Kerkhoff AD, Vogt M, Wood R. Clinical significance of lipoarabinomannan detection in urine using a low-cost point-of-care diagnostic assay for HIV-associated tuberculosis. AIDS 2012;26(13):1635-43. - PubMed
Lawn 2013 {published data only}
Lawn 2015 {published data only}
-
- Lawn SD, Kerkhoff AD, Burton R, Schutz C, Wyk G, Vogt M, et al. Rapid microbiological screening for tuberculosis in HIV-positive patients on the first day of acute hospital admission by systematic testing of urine samples using Xpert MTB/RIF: a prospective cohort in South Africa. BMC Medicine 2015;13:192. - PMC - PubMed
Lawn 2017 {published data only}
-
- Lawn SD, Kerkhoff AD, Burton R, Schutz C, Boulle A, Vogt M, et al. Diagnostic accuracy, incremental yield and prognostic value of Determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Medicine 2017;15(1):67. - PMC - PubMed
Lebina 2016 {published data only}
Lima 2020 {published data only}
Luo 2019 {published data only}
-
- Luo J, Luo M, Li J, Yu J, Yang H, Yi X, et al. Rapid direct drug susceptibility testing of Mycobacterium tuberculosis based on culture droplet digital polymerase chain reaction. International Journal of Tuberculosis and Lung Disease 2019;23(2):219-25. - PubMed
Maria 2018 {published data only}
-
- Maria MC, Rosarys MR, Misleidis SA, Grechen GL, Secretario CT, Raul DR. Diagnostic importance of "GeneXpert Mtb-Rif" in patients infected by the human immunodeficiency virus (HIV) [Spanish]. Archivos Venezolanos de Farmacologia y Terapeutica 2018;37(4):355-8.
Marks 2019 {published data only}
-
- Marks GB, Nguyen NV, Nguyen PT, Nguyen TA, Nguyen HB, Tran KH, et al. Community-wide screening for tuberculosis in a high-prevalence setting. New England Journal of Medicine 2019;381(14):1347-57. - PubMed
Marlowe 2011 {published data only}
Mbatchou 2019 {published data only}
-
- Mbatchou Ngahane B, Gaping Simen S, Halle M, Okalla C, Goupeyou Wandji IA. Prevalence of tuberculosis and its factors among patients on maintenance dialysis in Douala, Cameroon. American Journal of Respiratory and Critical Care Medicine. Conference 2019;201(Meeting abstracts):A5146.
Mbu 2018 {published data only}
-
- Mbu ET, Sauter F, Zoufaly A, Bronsvoort BM, Morgan KL, Noeske J, et al. Tuberculosis in people newly diagnosed with HIV at a large HIV care and treatment center in Northwest Cameroon: Burden, comparative screening and diagnostic yields, and patient outcomes. PLOS One 2018;13(6):e0199634. - PMC - PubMed
Meng 2017 {published data only}
Metcalfe 2015 {published data only}
Metcalfe 2016 {published data only}
Miller 2011 {published data only}
Mishra 2020 {published data only}
-
- Mishra H, Reeve BW, Palmer Z, Caldwell J, Dolby T, Naidoo CC. Xpert MTB/RIF Ultra and Xpert MTB/RIF for diagnosis of tuberculosis in an HIV-endemic setting with a high burden of previous tuberculosis: a two-cohort diagnostic accuracy study. Lancet Respiratory Medicine 2020;8(4):368-82. - PubMed
Modi 2016 {published data only}
Morishita 2017 {published data only}
Nathavitharana 2017 {published data only}
Nicol 2018 {published data only}
-
- Nicol MP, Workman L, Prins M, Bateman L, Ghebrekristos Y, Mbhele S, et al. Accuracy of Xpert MTB/RIF Ultra for the diagnosis of pulmonary tuberculosis in children. Pediatric Infectious Disease Journal 2018;37(10):e261-e3. - PubMed
Nikolayevskyy 2019 {published data only}
-
- Nikolayevskyy V, Kontsevaya I, Nikolaevskaya E, Surkova E, Samchenko S, Esipenko S. Diagnostic performance and impact of routinely implemented Xpert(R) MTB/RIF assay in a setting of high incidence of drug-resistant TB in Odessa Oblast, Ukraine. In: Clinical Microbiology and Infection. Vol. 25. 2019:1040.e1-1040.e6. - PubMed
Ou 2019 {published data only}
-
- Ou XC, Li H, Liu DX, Xia H, Ma XG, Wang SH, et al. Comparison of Xpert MTB/RIF, RealAmp, and CPA tests in detecting Mycobacterium tuberculosis. Biomedical and Environmental Sciences 2019;32(3):215-9. - PubMed
Ozkutuk 2014 {published data only}
-
- Ozkutuk N, Surucuoglu S. [Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary tuberculosis in an intermediate-prevalence setting]. Mikrobiyoloji Bulteni 2014;48(2):223-32. - PubMed
Parcell 2017 {published data only}
-
- Parcell BJ, Jarchow-MacDonald AA, Seagar AL, Laurenson IF, Prescott GJ, Lockhart M. Three year evaluation of Xpert MTB/RIF in a low prevalence tuberculosis setting: a Scottish perspective. Journal of Infection 2017;74(5):466-72. - PubMed
Park 2013 {published data only}
Pimkina 2015 {published data only}
-
- Pimkina E, Zablockis R, Nikolayevskyy V, Danila E, Davidaviciene E. The Xpert(R) MTB/RIF assay in routine diagnosis of pulmonary tuberculosis: a multicentre study in Lithuania. Respiratory Medicine 2015;109(11):1484-9. - PubMed
Ramamurthy 2016 {published data only}
-
- Ramamurthy K, Bhat S, Shenoy S, Rangnekar A. Xpert Mycobacterium tuberculosis/rifampicin assay: a boon in tuberculosis diagnostics. Asian Journal of Pharmaceutical and Clinical Research 2016;9(5):225-7.
Reepalu 2016 {published data only}
Reis 2019 {published data only}
-
- Reis AJ, Diniz JL, Silva AB, Silveira J, Basso R, Vieira R, et al. Laboratory tools for tuberculosis control in a setting with a high burden of HIV/AIDS. Journal of Medical Microbiology 2019;68(11):1622-8. - PubMed
Sarinoglu 2020 {published data only}
-
- Sarinoglu RC, Duman N, Unlu N, Yildizeli SO, Yagci AK. Xpert MTB/Ultra assay: handle with care. Journal of Infection 2020;80(3):350-71. - PubMed
Semitala 2019 {published data only}
Shah 2019 {published data only}
-
- Shah I, Bhamre R, Shetty NS. Accuracy of Xpert Mycobacterium tuberculosis/rifampicin assay in diagnosis of pulmonary tuberculosis. Infectious Diseases 2019;51(7):550-3. - PubMed
Sun 2019 {published data only}
-
- Sun DF, Zheng LL, Jin F, Wang MS. Evaluation of the double sputum Xpert tests for the diagnosis of pulmonary tuberculosis. Infectious Diseases 2019;51(7):541-2. - PubMed
Teo 2011 {published data only}
-
- Teo J, Jureen R, Chiang D, Chan D, Lin R. Comparison of two nucleic acid amplification assays, the Xpert MTB/RIF assay and the amplified Mycobacterium tuberculosis direct assay, for detection of Mycobacterium tuberculosis in respiratory and nonrespiratory specimens. Journal of Clinical Microbiology 2011;49(10):3659-62. - PMC - PubMed
Trajman 2014 {published data only}
-
- Trajman A, Durovni B, Saraceni V, Cordeiro-Santos M, Cobelens F, den Hof S. High positive predictive value of Xpert in a low rifampicin resistance prevalence setting. European Respiratory Journal 2014;44(6):1711-3. - PubMed
van Kampen 2015 {published data only}
Van Rie 2011 {published data only}
-
- Van Rie A. A single Xpert MTB/RIF test of sputum for diagnosis of tuberculosis and multidrug resistance shows high sensitivity and specificity and reduces diagnosis and treatment delays. Evidence-Based Medicine 2011;16(6):174-5. - PubMed
Yasemin 2019 {published data only}
-
- Yasemin A, Ahmad S, Afzal S, Ullah A, Sheed A. Evaluation of GeneXpert MTB/RIF assay for detection of pulmonary tuberculosis on sputum samples. Journal of College of Physicians and Surgeons Pakistan 2019;29(1):66-9. - PubMed
Yoon 2019 {published data only}
Additional references
Balshem 2011
-
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. - PubMed
Banada 2010
Bjerrum 2019
-
- Bjerrum S, Schiller I, Dendukuri N, Kohli M, Nathavitharana RR, Zwerling AA, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database of Systematic Reviews 2019, Issue 10. Art. No: CD011420. [DOI: 10.1002/14651858.CD011420.pub3] - DOI - PMC - PubMed
Broger 2020
Cain 2010
-
- Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. New England Journal of Medicine 2010;362:707-16. - PubMed
Cepheid 2018
-
- Cepheid. Brochure: Xpert® MTB/RIF Ultra. https://www.cepheid.com/en/tests/Critical-Infectious-Diseases/Xpert-MTB-... (accessed 21 January 2021).
Cepheid 2019
-
- Cepheid. Brochure: Xpert® MTB/RIF assay. Xpert-MTB-RIF-ENGLISH-Package-Insert-301-1404-Rev-F.pdf (accessed 20 January 2021).
Chakravorty 2017
Chu 2006
-
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331-2. - PubMed
Cochrane Special Collection 2020
-
- Cochrane Infectious Diseases Group. Diagnosing tuberculosis. www.cochranelibrary.com/collections/doi/SC000034/full (accessed 20 January 2021).
Covidence [Computer program]
-
- Covidence systematic review software. Available at www.covidence.org. Melbourne, Australia: Veritas Health Innovation, (accessed 10 September 2020). Available at covidence.org.
Dorman 2018
Enos 2018
Ford 2016
Frascella 2020
-
- Frascella B, Richards AS, Sossen B, Emery JC, Odone A, Law I, et al. Subclinical tuberculosis disease - a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clinical Infectious Diseases 2020 Sept 16 [Epub ahead of print]:ciaa1402. [DOI: 10.1093/cid/ciaa1402] - DOI - PMC - PubMed
Getahun 2010
-
- Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clinical Infectious Diseases 2010;50 Suppl 3:S201–7. - PubMed
Global Laboratory Initiative 2019
-
- Global Laboratory Intiaitive. Practical guide to implementing a quality assurance system for Xpert MTB/RIF testing. www.stoptb.org/wg/gli/assets/documents/Xpert-QA-guide-2019.pdf (accessed 7 January 2020).
GRADEpro GDT 2020 [Computer program]
-
- GRADEpro GDT. Version accessed after 12 June 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Gunasekera 2020
-
- Gunasekera K, Cohen T, Gao W, Ayles H, Godfrey-Faussett P, Claessens M. Smoking and HIV associated with subclinical tuberculosis: analysis of a population-based prevalence survey. International Journal of Tuberculosis and Lung Disease 2020;24(3):340-346. - PubMed
Hamada 2018
-
- Hamada Y, Lujan J, Schenkel K, Ford N, Getahun H. Sensitivity and specificity of WHO's recommended four-symptom screening rule for tuberculosis in people living with HIV: a systematic review and meta-analysis. Lancet HIV 2018;5(9):e515-23. - PubMed
Harris 2019
Horne 2019
-
- Horne DJ, Kohli M, Zifodya JS, Schiller I, Dendukuri N, Tollefson D, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD009593. [DOI: 10.1002/14651858.CD009593.pub4] - DOI - PMC - PubMed
Lewinsohn 2017
-
- Lewinsohn DM, Leonard MK, LoBue PA, Cohn DL, Daley CL, Desmond E, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: diagnosis of tuberculosis in adults and children. Clinical Infectious Diseases 2017;64(2):e1-e33. [PMID: ] - PubMed
Lunn 2009
-
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique, and future directions. Statistics in Medicine 2009;28(25):3049-67. - PubMed
Macaskill 2010
-
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editors, Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0. The Cochrane Collaboration, 2010. Available from srdta.cochrane.org.
Moher 2009
Nguyen 2020
Peter 2016
-
- Peter JG, Zijenah LS, Chanda D, Clowes P, Lesosky M, Gina P, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016;387(10024):1187-97. - PubMed
R Core Team 2019 [Computer program]
-
- R Core Team (2019). R: A language and environment for statistical computing. Version accessed after 12 June 2020. Vienna, Austria: R Foundation for Statistical Computing, 2019. Available at www.R-project.org.
Reitsma 2005
-
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982-90. - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Schünemann 2008
Schünemann 2016
-
- Schünemann HJ, Mustafa R, Brozek J, Santesso N, Alonso-Coello P, Guyatt G, et al. GRADE Working Group. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. Journal of Clinical Epidemiology 2016;76:89-98. [DOI: 10.1016/j.jclinepi.2016.01.032] - DOI - PubMed
Schünemann 2020a
-
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 1. Study design, risk of bias and indirectness in rating the certainty across a body of evidence for test accuracy. Journal of Clinical Epidemiology 2020;122:129-41. [DOI: 10.1016/j.jclinepi.2019.12.020] - DOI - PubMed
Schünemann 2020b
-
- Schünemann HJ, Mustafa R, Brozek J, Steingart KR, Leeflang M, Murad MH, et al. GRADE guidelines: 21 part 2. Inconsistency, Imprecision, publication bias and other domains for rating the certainty of evidence for test accuracy and presenting it in evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2020;122:142-52. [DOI: 10.1016/j.jclinepi.2019.12.021] - DOI - PubMed
Stata [Computer program]
-
- Stata. Version 15. College Station, TX, USA: StataCorp, 2017. Available at www.stata.com.
Steingart 2014
Unitaid 2017
-
- Boyle D. Tuberculosis Diagnostics Technology and Market Landscape. 5th edition. Vernier: World Health Organization Unitaid Secretariat, 2017.
UN Sustainable Development Goals 2030
-
- United Nations General Assembly. Transforming our world: the 2030 agenda for sustainable development. Resolution adopted by the General Assembly on 25 September 2015. https://sustainabledevelopment.un.org/post2015/transformingourworld/publ... (accessed 22 July 2020).
van't Hoog 2013
-
- van't Hoog AH, Langendam MW, Mitchell E, Cobelens FG, Sinclair D, Leeflang MM, et al. A systematic review of the sensitivity and specificity of symptom- and chest-radiography screening for active pulmonary tuberculosis in HIV-negative persons and persons with unknown HIV status. Report to the World Health Organization. 2013. who.int/tb/Review2Accuracyofscreeningtests.pdf?ua=1 (accessed 23 June 2020).
Whiting 2011
-
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529-36. - PubMed
WHO 2011
-
- World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF system for the diagnosis of pulmonary and extrapulmonary TB in adults and children. apps.who.int/iris/handle/10665/112472 (accessed 18 November 2020). - PubMed
WHO 2016
-
- World Health Organization. Xpert MTB/RIF assay for the diagnosis of TB: meeting report 2016. apps.who.int/iris/handle/10665/250383 (accessed 10 October 2020).
WHO Compendium of WHO guidelines 2018
-
- World Health Organanization. Compendium of WHO guidelines and associated standards: ensuring optimum delivery of the cascade of care for patients with tuberculosis. Second edition - June 2018. https://www.who.int/tb/publications/Compendium_WHO_guidelines_TB_2017/en/ (accessed 10 September 2020).
WHO Consolidated Guidelines (Module 3) 2020
-
- World Health Organization. World Health Organization. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection; June 2020. who.int/publications/i/item/who-consolidated-guidelines-on-tuberculosis-... (accessed 1 July 2020).
WHO Consolidated Guidelines (Module 4) 2020
-
- World Health Organization. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment. who.int/publications/i/item/9789240007048 (accessed 1 July 2020). - PubMed
WHO END TB 2015
-
- World Health Organization. Implementing the End TB Strategy: the essentials. WHO/HTM/TB/2015.31. who.int/tb/publications/2015/The_Essentials_to_End_TB/en/ (accessed 23 June 2020).
WHO Global TB Report 2019
-
- World Health Organization. Global Tuberculosis Report 2019. Geneva: World Health Organization, 2019.
WHO Global TB Report 2020
-
- World Health Organization. Global Tuberculosis Report 2020. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization, 2020.
WHO Guidelines 2016
-
- World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis, 2016 update. October 2016 revision. apps.who.int/iris/bitstream/handle/10665/250125/9789241549639-eng.pdf (accessed 25 November 2020).
WHO LPA 2016
-
- World Health Organization. The use of molecular line probe assays for the detection of resistance to isoniazid and rifampicin: policy update. WHO/HTM/TB/2016.12. Geneva: World Health Organization 2016.
WHO Rapid Communication 2020
-
- World Health Organization. Rapid communication on systematic screening for tuberculosis.. who.int/publications/i/item/rapid-communication-on-the-systematic-screen... (accessed 18 January 2021).
WHO Systematic screening 2013
-
- World Health Organization. Systematic screening for active tuberculosis: principles and recommendations. WHO/HTM/TB/2013.04. Geneva: World Health Organization, 2013. - PubMed
WHO Systematic screening 2015
-
- World Health Organization. Systematic screening for active tuberculosis: an operational guide WHO/HTM/TB/2015.16. who.int/tb/publications/systematic_screening/en/ (accessed 25 November 2020).
WHO Xpert MTB/RIF 2013
-
- World Health Organization. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children. https://apps.who.int/iris/handle/10665/112472 (accessed 23 June 2020). - PubMed
WHO Xpert Ultra 2017
-
- World Health Organization. WHO meeting report of a technical expert consultation: non-inferiority analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. WHO/HTM/TB/2017.04. Geneva: WHO 2017.
World Bank 2020
-
- World Bank. World bank list of economies. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-b... (accessed 18 November 2020).
Zellweger 2006
-
- Zellweger JP, Heinzer R, Touray M, Vidondo B, Altpeter E. Intra-observer and overall agreement in the radiological assessment of tuberculosis. International Journal of Tuberculosis and Lung Diseases 2006;10(10):1123-6. - PubMed
Zifodya 2021
-
- Zifodya JS, Kreniske JS, Schiller I, Kohli M, Dendukuri N, Schumacher SG, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD009593. [DOI: 10.1002/14651858.CD009593.pub5] - DOI - PMC - PubMed
References to other published versions of this review
Shapiro 2020
-
- Shapiro AE, Ross JM, Schiller I, Kohli M, Dendukuri N, Steingart KR, et al. Xpert MTB/RIF and Xpert Ultra assays for pulmonary tuberculosis and rifampicin resistance in adults irrespective of signs or symptoms of pulmonary tuberculosis. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013694. [DOI: 10.1002/14651858.CD013694] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

