Deficiency of cold-inducible RNA-binding protein exacerbated monocrotaline-induced pulmonary artery hypertension through Caveolin1 and CAVIN1
- PMID: 33755319
- PMCID: PMC8107102
- DOI: 10.1111/jcmm.16437
Deficiency of cold-inducible RNA-binding protein exacerbated monocrotaline-induced pulmonary artery hypertension through Caveolin1 and CAVIN1
Abstract
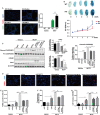
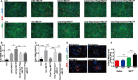
Cold-inducible RNA-binding protein (CIRP) was a crucial regulator in multiple diseases. However, its role in pulmonary artery hypertension (PAH) is still unknown. Here, we first established monocrotaline (MCT)-induced rat PAH model and discovered that CIRP was down-regulated predominantly in the endothelium of pulmonary artery after MCT injection. We then generated Cirp-knockout (Cirp-KO) rats, which manifested severer PAH with exacerbated endothelium damage in response to MCT. Subsequently, Caveolin1 (Cav1) and Cavin1 were identified as downstream targets of CIRP in MCT-induced PAH, and the decreased expression of these two genes aggravated the injury and apoptosis of pulmonary artery endothelium. Moreover, CIRP deficiency intensified monocrotaline pyrrole (MCTP)-induced rat pulmonary artery endothelial cells (rPAECs) injuries both in vivo and in vitro, which was counteracted by Cav1 or Cavin1 overexpression. In addition, CIRP regulated the proliferative effect of conditioned media from MCTP-treated rPAECs on rat pulmonary artery smooth muscle cells, which partially explained the exceedingly thickened pulmonary artery intimal media in Cirp-KO rats after MCT treatment. These results demonstrated that CIRP acts as a critical protective factor in MCT-induced rat PAH by directly regulating CAV1 and CAVIN1 expression, which may facilitate the development of new therapeutic targets for the intervention of PAH.
Keywords: CAVIN1; Caveolin1; cold-inducible RNA-binding protein; monocrotaline; pulmonary artery endothelial cells; pulmonary artery hypertension.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures



Similar articles
-
Protection against monocrotaline-induced pulmonary arterial hypertension and caveolin-1 downregulation by fluvastatin in rats.Mol Med Rep. 2018 Mar;17(3):3944-3950. doi: 10.3892/mmr.2017.8345. Epub 2017 Dec 22. Mol Med Rep. 2018. PMID: 29286128
-
Calcineurin/NFAT Signaling Modulates Pulmonary Artery Smooth Muscle Cell Proliferation, Migration and Apoptosis in Monocrotaline-Induced Pulmonary Arterial Hypertension Rats.Cell Physiol Biochem. 2018;49(1):172-189. doi: 10.1159/000492852. Epub 2018 Aug 22. Cell Physiol Biochem. 2018. PMID: 30134231
-
miR-181a/b-5p ameliorates inflammatory response in monocrotaline-induced pulmonary arterial hypertension by targeting endocan.J Cell Physiol. 2020 May;235(5):4422-4433. doi: 10.1002/jcp.29318. Epub 2019 Oct 21. J Cell Physiol. 2020. PMID: 31637717
-
Lung vascular injury from monocrotaline pyrrole, a putative hepatic metabolite.Adv Exp Med Biol. 1991;283:477-87. doi: 10.1007/978-1-4684-5877-0_64. Adv Exp Med Biol. 1991. PMID: 1906225 Review.
-
The role of Cold-Inducible RNA-binding protein in respiratory diseases.J Cell Mol Med. 2022 Feb;26(4):957-965. doi: 10.1111/jcmm.17142. Epub 2021 Dec 24. J Cell Mol Med. 2022. PMID: 34953031 Free PMC article. Review.
Cited by
-
Identification of biomarkers, pathways, and potential therapeutic targets for heart failure using next-generation sequencing data and bioinformatics analysis.Ther Adv Cardiovasc Dis. 2023 Jan-Dec;17:17539447231168471. doi: 10.1177/17539447231168471. Ther Adv Cardiovasc Dis. 2023. PMID: 37092838 Free PMC article.
-
Molecular regulation and therapeutic implications of cell death in pulmonary hypertension.Cell Death Discov. 2023 Jul 12;9(1):239. doi: 10.1038/s41420-023-01535-6. Cell Death Discov. 2023. PMID: 37438344 Free PMC article. Review.
-
Identification of the shared hub gene signatures and molecular mechanisms between HIV-1 and pulmonary arterial hypertension.Sci Rep. 2024 Mar 25;14(1):7048. doi: 10.1038/s41598-024-55645-x. Sci Rep. 2024. PMID: 38528047 Free PMC article.
-
A role of TRIM59 in pulmonary hypertension: modulating the protein ubiquitylation modification.J Transl Med. 2023 Nov 17;21(1):821. doi: 10.1186/s12967-023-04712-4. J Transl Med. 2023. PMID: 37978515 Free PMC article.
-
Differential responses of pulmonary vascular cells from PAH patients and controls to TNFα and the effect of the BET inhibitor JQ1.Respir Res. 2023 Jul 29;24(1):193. doi: 10.1186/s12931-023-02499-y. Respir Res. 2023. PMID: 37516840 Free PMC article.
References
-
- Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4(4):306‐322. - PubMed
-
- Kiely DG, Elliot CA, Sabroe I, Condliffe R. Pulmonary hypertension: diagnosis and management. BMJ. 2013;346:f2028. - PubMed
-
- MacIver DH, Adeniran I, MacIver IR, Revell A, Zhang H. Physiological mechanisms of pulmonary hypertension. Am Heart J. 2016;180:1‐11. - PubMed
-
- Alan B, Nalbantgil S. Genetic, cellular and molecular mechanisms of pulmonary arterial hypertension. Anadolu Kardiyoloji Derg/Anatolian J Cardiol. 2010;10(Suppl 1):9‐13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

