Pathogenic variants in TNNC2 cause congenital myopathy due to an impaired force response to calcium
- PMID: 33755597
- PMCID: PMC8087209
- DOI: 10.1172/JCI145700
Pathogenic variants in TNNC2 cause congenital myopathy due to an impaired force response to calcium
Abstract
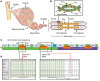
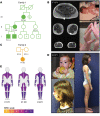
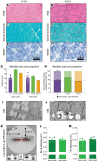
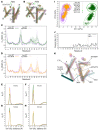
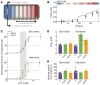
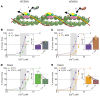
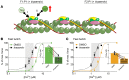
Troponin C (TnC) is a critical regulator of skeletal muscle contraction; it binds Ca2+ to activate muscle contraction. Surprisingly, the gene encoding fast skeletal TnC (TNNC2) has not yet been implicated in muscle disease. Here, we report 2 families with pathogenic variants in TNNC2. Patients present with a distinct, dominantly inherited congenital muscle disease. Molecular dynamics simulations suggested that the pathomechanisms by which the variants cause muscle disease include disruption of the binding sites for Ca2+ and for troponin I. In line with these findings, physiological studies in myofibers isolated from patients' biopsies revealed a markedly reduced force response of the sarcomeres to [Ca2+]. This pathomechanism was further confirmed in experiments in which contractile dysfunction was evoked by replacing TnC in myofibers from healthy control subjects with recombinant, mutant TnC. Conversely, the contractile dysfunction of myofibers from patients was repaired by replacing endogenous, mutant TnC with recombinant, wild-type TnC. Finally, we tested the therapeutic potential of the fast skeletal muscle troponin activator tirasemtiv in patients' myofibers and showed that the contractile dysfunction was repaired. Thus, our data reveal that pathogenic variants in TNNC2 cause congenital muscle disease, and they provide therapeutic angles to repair muscle contractility.
Keywords: Calcium signaling; Genetic diseases; Genetics; Muscle Biology; Neuromuscular disease.
Conflict of interest statement
Figures

Similar articles
-
Troponin Variants in Congenital Myopathies: How They Affect Skeletal Muscle Mechanics.Int J Mol Sci. 2021 Aug 25;22(17):9187. doi: 10.3390/ijms22179187. Int J Mol Sci. 2021. PMID: 34502093 Free PMC article. Review.
-
Pathogenic TNNI1 variants disrupt sarcomere contractility resulting in hypo- and hypercontractile muscle disease.Sci Transl Med. 2024 Apr 3;16(741):eadg2841. doi: 10.1126/scitranslmed.adg2841. Epub 2024 Apr 3. Sci Transl Med. 2024. PMID: 38569017
-
The low-affinity Ca2(+)-binding sites in cardiac/slow skeletal muscle troponin C perform distinct functions: site I alone cannot trigger contraction.Proc Natl Acad Sci U S A. 1990 Dec;87(24):9538-42. doi: 10.1073/pnas.87.24.9538. Proc Natl Acad Sci U S A. 1990. PMID: 2263608 Free PMC article.
-
The effect of pH on the Ca2+ affinity of the Ca2+ regulatory sites of skeletal and cardiac troponin C in skinned muscle fibres.J Muscle Res Cell Motil. 1997 Oct;18(5):599-609. doi: 10.1023/a:1018623604365. J Muscle Res Cell Motil. 1997. PMID: 9350012
-
Biochemical characterisation of Troponin C mutations causing hypertrophic and dilated cardiomyopathies.J Muscle Res Cell Motil. 2014 Apr;35(2):161-78. doi: 10.1007/s10974-014-9382-0. Epub 2014 Apr 18. J Muscle Res Cell Motil. 2014. PMID: 24744096 Review.
Cited by
-
Pathogenic TNNT1 variants are associated with aberrant thin filament compliance and myofibre hyper-contractility.J Physiol. 2025 Jun;603(12):3533-3550. doi: 10.1113/JP288109. Epub 2025 May 5. J Physiol. 2025. PMID: 40320982 Free PMC article.
-
Troponin Variants in Congenital Myopathies: How They Affect Skeletal Muscle Mechanics.Int J Mol Sci. 2021 Aug 25;22(17):9187. doi: 10.3390/ijms22179187. Int J Mol Sci. 2021. PMID: 34502093 Free PMC article. Review.
-
MYL1-Related Congenital Myopathy: Clinical, Genetic and Pathological Insights.Neuropathol Appl Neurobiol. 2025 Jun;51(3):e70025. doi: 10.1111/nan.70025. Neuropathol Appl Neurobiol. 2025. PMID: 40488356 Free PMC article.
-
Elucidating the molecular basis of spontaneous activation in an engineered mechanosensitive channel.Comput Struct Biotechnol J. 2022 May 23;20:2539-2550. doi: 10.1016/j.csbj.2022.05.022. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 35685356 Free PMC article.
-
Structural Changes beyond the EF-Hand Contribute to Apparent Calcium Binding Affinities: Insights from Parvalbumins.J Phys Chem B. 2021 Jun 24;125(24):6390-6405. doi: 10.1021/acs.jpcb.1c01269. Epub 2021 Jun 11. J Phys Chem B. 2021. PMID: 34115511 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

