Nuclear DEK preserves hematopoietic stem cells potential via NCoR1/HDAC3-Akt1/2-mTOR axis
- PMID: 33755722
- PMCID: PMC7992411
- DOI: 10.1084/jem.20201974
Nuclear DEK preserves hematopoietic stem cells potential via NCoR1/HDAC3-Akt1/2-mTOR axis
Abstract
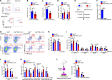
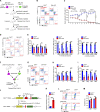
The oncogene DEK is found fused with the NUP214 gene creating oncoprotein DEK-NUP214 that induces acute myeloid leukemia (AML) in patients, and secreted DEK protein functions as a hematopoietic cytokine to regulate hematopoiesis; however, the intrinsic role of nuclear DEK in hematopoietic stem cells (HSCs) remains largely unknown. Here, we show that HSCs lacking DEK display defects in long-term self-renew capacity, eventually resulting in impaired hematopoiesis. DEK deficiency reduces quiescence and accelerates mitochondrial metabolism in HSCs, in part, dependent upon activating mTOR signaling. At the molecular level, DEK recruits the corepressor NCoR1 to repress acetylation of histone 3 at lysine 27 (H3K27ac) and restricts the chromatin accessibility of HSCs, governing the expression of quiescence-associated genes (e.g., Akt1/2, Ccnb2, and p21). Inhibition of mTOR activity largely restores the maintenance and potential of Dek-cKO HSCs. These findings highlight the crucial role of nuclear DEK in preserving HSC potential, uncovering a new link between chromatin remodelers and HSC homeostasis, and have clinical implications.
© 2021 Chen et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures









Similar articles
-
Inhibition of DEK restores hematopoietic stem cell function in Fanconi anemia.J Exp Med. 2025 Mar 3;222(3):e20241248. doi: 10.1084/jem.20241248. Epub 2025 Jan 21. J Exp Med. 2025. PMID: 39836085
-
Secreted nuclear protein DEK regulates hematopoiesis through CXCR2 signaling.J Clin Invest. 2019 May 20;129(6):2555-2570. doi: 10.1172/JCI127460. eCollection 2019 May 20. J Clin Invest. 2019. PMID: 31107242 Free PMC article.
-
DEK regulates hematopoietic stem engraftment and progenitor cell proliferation.Stem Cells Dev. 2012 Jun 10;21(9):1449-54. doi: 10.1089/scd.2011.0451. Epub 2011 Oct 27. Stem Cells Dev. 2012. PMID: 21943234 Free PMC article.
-
A role for intracellular and extracellular DEK in regulating hematopoiesis.Curr Opin Hematol. 2017 Jul;24(4):300-306. doi: 10.1097/MOH.0000000000000344. Curr Opin Hematol. 2017. PMID: 28306668 Free PMC article. Review.
-
The impact of the chromatin binding DEK protein in hematopoiesis and acute myeloid leukemia.Exp Hematol. 2023 Jul;123:18-27. doi: 10.1016/j.exphem.2023.05.002. Epub 2023 May 10. Exp Hematol. 2023. PMID: 37172756 Free PMC article. Review.
Cited by
-
The role of protein acetylation in carcinogenesis and targeted drug discovery.Front Endocrinol (Lausanne). 2022 Sep 12;13:972312. doi: 10.3389/fendo.2022.972312. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36171897 Free PMC article. Review.
-
Molecular regulation of hematopoietic stem cell quiescence.Cell Mol Life Sci. 2022 Mar 31;79(4):218. doi: 10.1007/s00018-022-04200-w. Cell Mol Life Sci. 2022. PMID: 35357574 Free PMC article. Review.
-
Frontier progress and translational challenges of pluripotent differentiation of stem cells.Front Genet. 2025 Apr 28;16:1583391. doi: 10.3389/fgene.2025.1583391. eCollection 2025. Front Genet. 2025. PMID: 40357368 Free PMC article. Review.
-
Targeting KPNB1 suppresses AML cells by inhibiting HMGB2 nuclear import.Oncogene. 2025 Jun;44(21):1646-1661. doi: 10.1038/s41388-025-03340-0. Epub 2025 Mar 13. Oncogene. 2025. PMID: 40082556
-
Inhibition of DEK restores hematopoietic stem cell function in Fanconi anemia.J Exp Med. 2025 Mar 3;222(3):e20241248. doi: 10.1084/jem.20241248. Epub 2025 Jan 21. J Exp Med. 2025. PMID: 39836085
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

