Natural Glycoforms of Human Interleukin 6 Show Atypical Plasma Clearance
- PMID: 33756033
- PMCID: PMC8251587
- DOI: 10.1002/anie.202101496
Natural Glycoforms of Human Interleukin 6 Show Atypical Plasma Clearance
Abstract
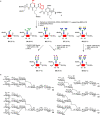
A library of glycoforms of human interleukin 6 (IL-6) comprising complex and mannosidic N-glycans was generated by semisynthesis. The three segments were connected by sequential native chemical ligation followed by two-step refolding. The central glycopeptide segments were assembled by pseudoproline-assisted Lansbury aspartylation and subsequent enzymatic elongation of complex N-glycans. Nine IL-6 glycoforms were synthesized, seven of which were evaluated for in vivo plasma clearance in rats and compared to non-glycosylated recombinant IL-6 from E. coli. Each IL-6 glycoform was tested in three animals and reproducibly showed individual serum clearances depending on the structure of the N-glycan. The clearance rates were atypical, since the 2,6-sialylated glycoforms of IL-6 cleared faster than the corresponding asialo IL-6 with terminal galactoses. Compared to non-glycosylated IL-6 the plasma clearance of IL-6 glycoforms was delayed in the presence of larger and multibranched N-glycans in most cases.
Keywords: glycopeptides; glycoproteins; native chemical ligation; oligosaccharides; serum clearance.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Wolf J., Rose-John S., Garbers C., Cytokine 2014, 70, 11–20. - PubMed
-
- Parekh R. B., Dwek R. A., Rademacher T. W., Opdenakker G., Van Damme J., Eur. J. Biochem. 1992, 203, 135–141. - PubMed
-
- Unverzagt C., Kajihara Y., Chem. Soc. Rev. 2013, 42, 4408–4420. - PubMed
-
- Reif A., Siebenhaar S., Tröster A., Schmälzlein M., Lechner C., Velisetty P., Gottwald K., Pöhner C., Boos I., Schubert V., Rose-John S., Unverzagt C., Angew. Chem. Int. Ed. 2014, 53, 12125–12131; - PubMed
- Angew. Chem. 2014, 126, 12321–12327.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

