Phytochrome Signaling Networks
- PMID: 33756095
- PMCID: PMC10988782
- DOI: 10.1146/annurev-arplant-080620-024221
Phytochrome Signaling Networks
Abstract
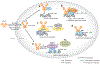
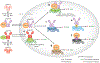
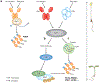
The perception of light signals by the phytochrome family of photoreceptors has a crucial influence on almost all aspects of growth and development throughout a plant's life cycle. The holistic regulatory networks orchestrated by phytochromes, including conformational switching, subcellular localization, direct protein-protein interactions, transcriptional and posttranscriptional regulations, and translational and posttranslational controls to promote photomorphogenesis, are highly coordinated and regulated at multiple levels. During the past decade, advances using innovative approaches have substantially broadened our understanding of the sophisticated mechanisms underlying the phytochrome-mediated light signaling pathways. This review discusses and summarizes these discoveries of the role of the modular structure of phytochromes, phytochrome-interacting proteins, and their functions; the reciprocal modulation of both positive and negative regulators in phytochrome signaling; the regulatory roles of phytochromes in transcriptional activities, alternative splicing, and translational regulation; and the kinases and E3 ligases that modulate PHYTOCHROME INTERACTING FACTORs to optimize photomorphogenesis.
Keywords: E3 ligase; PHYTOCHROME INTERACTING FACTOR; PIF; kinase; light signaling; light-regulated alternative splicing; phytochrome.
Figures
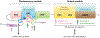


References
-
- Ádám É, Kircher S, Liu P, Mérai Z, González-Schain N, et al. 2013. Comparative functional analysis of full-length and N-terminal fragments of phytochrome C, D and E in red light-induced signaling. New Phytol. 200:86–96 - PubMed
-
- Al-Sady B, Ni W, Kircher S, Schafer E, Quail PH. 2006. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol. Cell 23:439–46 - PubMed
-
- Bae G, Choi G. 2008. Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol 59:281–311 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

