Caution in interpretation of SARS-CoV-2 quantification based on RT-PCR cycle threshold value
- PMID: 33756311
- PMCID: PMC7929791
- DOI: 10.1016/j.diagmicrobio.2021.115366
Caution in interpretation of SARS-CoV-2 quantification based on RT-PCR cycle threshold value
Abstract
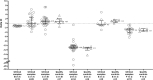
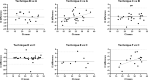
RT-PCR is the reference method for diagnosis of a Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) infection. During the setting up of 6 SARS-CoV-2 RT-PCR assays in our laboratory, comparative evaluations were systematically undertaken and allowed to evidence major discrepancies on cycle threshold RT-PCR results between techniques. These tendencies were confirmed in routine application when analyzing sequential samples from the same patients. Our aim was to examine the impact of the technique among factors influencing RT-PCR result, a far surrogate of 'viral load' in the heterogeneous environment of respiratory specimens.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures

References
-
- Pan Y, Long L, Zhang D, Yuan T, Cui S, Yang P, et al. Potential false-negative nucleic acid testing results for severe acute respiratory syndrome coronavirus 2 from thermal inactivation of samples with low viral loads. Clin Chem. 2020;66(6):794–801. doi: 10.1093/clinchem/hvaa091. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

