Ultrasound-mediated gene delivery into suspended plant cells using polyethyleneimine-coated mesoporous silica nanoparticles
- PMID: 33756435
- PMCID: PMC7994536
- DOI: 10.1016/j.ultsonch.2021.105507
Ultrasound-mediated gene delivery into suspended plant cells using polyethyleneimine-coated mesoporous silica nanoparticles
Abstract
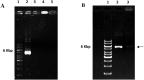
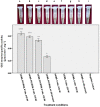
Sonoporation, ultrasound-mediated membrane perforation can potentially puncture plasma membrane and rigid cell wall on presumably reversible basis which benefit gene transfection and plant biotechnology. Herein, positively charged poly-ethyleneimine (PEI)-coated mesoporous silica nanoparticles (MSNs) with an average diameter of 100 ± 8.7 nm was synthesized for GUS-encoding plasmid delivery into the suspended tobacco cells using the ultrasound treatment. The overall potential of PEI-MSN for DNA adsorption was measured at 43.43 μg DNA mg-1 PEI-MSNs. It was shown that high level of sonoporation may adversely upset the cell viability. Optimal conditions of ultrasonic treatment are obtained as 8 min at 3 various intensities of 160, 320 and 640 W. Histochemical staining assay was used to follow the protein expression. It was shown that PEI-coated MSNs efficiently transfer the GUS-encoding plasmid DNA into the tobacco cells. The results of this study showed that ultrasonic treatment provides an economical and straightforward approach for gene transferring into the plant cells without any need to complicated devices and concerns about safety issues.
Keywords: GUS-encoding plasmid DNA; Gene transfection; Mesoporous silica nanoparticles; Ultrasonic treatment.
Copyright © 2021 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Enhanced gene delivery by polyethyleneimine coated mesoporous silica nanoparticles.Pharm Dev Technol. 2019 Jan;24(1):127-132. doi: 10.1080/10837450.2018.1431930. Epub 2018 Feb 6. Pharm Dev Technol. 2019. PMID: 29357725
-
Stimuli-responsive hybrid nanocarriers developed by controllable integration of hyperbranched PEI with mesoporous silica nanoparticles for sustained intracellular siRNA delivery.Int J Nanomedicine. 2016 Dec 8;11:6591-6608. doi: 10.2147/IJN.S120611. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27994460 Free PMC article.
-
The packaging of siRNA within the mesoporous structure of silica nanoparticles.Biomaterials. 2011 Dec;32(35):9546-56. doi: 10.1016/j.biomaterials.2011.08.068. Epub 2011 Sep 8. Biomaterials. 2011. PMID: 21906804
-
Mesoporous silica nanoparticles for therapeutic/diagnostic applications.Biomed Pharmacother. 2019 Jan;109:1100-1111. doi: 10.1016/j.biopha.2018.10.167. Epub 2018 Nov 6. Biomed Pharmacother. 2019. PMID: 30551360 Review.
-
Mesoporous Silica Nanoparticles as a Carrier Platform for Intracellular Delivery of Nucleic Acids.Biochemistry (Mosc). 2017 Jun;82(6):655-662. doi: 10.1134/S0006297917060025. Biochemistry (Mosc). 2017. PMID: 28601075 Review.
Cited by
-
Nanotechnology in Food and Plant Science: Challenges and Future Prospects.Plants (Basel). 2023 Jul 6;12(13):2565. doi: 10.3390/plants12132565. Plants (Basel). 2023. PMID: 37447126 Free PMC article. Review.
-
Electrically controlled mRNA delivery using a polypyrrole-graphene oxide hybrid film to promote osteogenic differentiation of human mesenchymal stem cells.Nano Res. 2022;15(10):9253-9263. doi: 10.1007/s12274-022-4613-y. Epub 2022 Jul 23. Nano Res. 2022. PMID: 35911478 Free PMC article.
-
Plant biomacromolecule delivery methods in the 21st century.Front Genome Ed. 2022 Oct 14;4:1011934. doi: 10.3389/fgeed.2022.1011934. eCollection 2022. Front Genome Ed. 2022. PMID: 36311974 Free PMC article. Review.
-
Chitosan-Modified Polyethyleneimine Nanoparticles for Enhancing the Carboxylation Reaction and Plants' CO2 Uptake.ACS Nano. 2023 Feb 28;17(4):3430-3441. doi: 10.1021/acsnano.2c09255. Epub 2023 Feb 16. ACS Nano. 2023. PMID: 36796108 Free PMC article.
-
The role of nanoparticles in transforming plant genetic engineering: advancements, challenges and future prospects.Funct Integr Genomics. 2025 Jan 22;25(1):23. doi: 10.1007/s10142-025-01528-x. Funct Integr Genomics. 2025. PMID: 39841261 Review.
References
-
- Prasad B.D., Ranjan T., Sahni S., Sharma V. The recent advances in transgenic crops: a review. J. Cell Tissue Res. 2017;17:6185–6196.
-
- Kościańska E., Wypijewski K. Electroporated intact BY-2 tobacco culture cells as a model of transient expression study. Acta Biochim. Pol. 2001;48(3):657–661. - PubMed
-
- Prasanna G.L., Panda T. Electroporation: basic principles, practical considerations and applications in molecular biology. Bioprocess Eng. 1997;16:261–264.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

