Phase 1 Safety Trial of Autologous Human Schwann Cell Transplantation in Chronic Spinal Cord Injury
- PMID: 33757304
- PMCID: PMC9360180
- DOI: 10.1089/neu.2020.7590
Phase 1 Safety Trial of Autologous Human Schwann Cell Transplantation in Chronic Spinal Cord Injury
Abstract
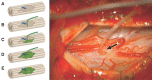
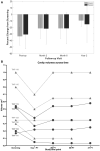
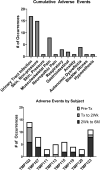
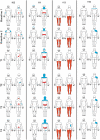
A phase 1 open-label, non-randomized clinical trial was conducted to determine feasibility and safety of autologous human Schwann cell (ahSC) transplantation accompanied by rehabilitation in participants with chronic spinal cord injury (SCI). Magnetic resonance imaging (MRI) was used to screen eligible participants to estimate an individualized volume of cell suspension to be implanted. The trial incorporated standardized multi-modal rehabilitation before and after cell delivery. Participants underwent sural nerve harvest, and ahSCs were isolated and propagated in culture. The dose of culture-expanded ahSCs injected into the chronic spinal cord lesion of each individual followed a cavity-filling volume approach. Primary outcome measures for safety and trend-toward efficacy were assessed. Two participants with American Spinal Injury Association Impairment Scale (AIS) A and two participants with incomplete chronic SCI (AIS B, C) were each enrolled in cervical and thoracic SCI cohorts (n = 8 total). All participants completed the study per protocol, and no serious adverse events related to sural nerve harvest or ahSC transplantation were reported. Urinary tract infections and skin abrasions were the most common adverse events reported. One participant experienced a 4-point improvement in motor function, a 6-point improvement in sensory function, and a 1-level improvement in neurological level of injury. Follow-up MRI in the cervical (6 months) and thoracic (24 months) cohorts revealed a reduction in cyst volume after transplantation with reduced effect over time. This phase 1 trial demonstrated the feasibility and safety of ahSC transplantation combined with a multi-modal rehabilitation protocol for participants with chronic SCI.
Keywords: Schwann cells; autologous transplantation; chronic; human; paraplegia; spinal cord injury.
Conflict of interest statement
Allan D. Levi:Grant support - Department of Defense (DOD), NIH/NINDS – R25 / R21
Teaching Honorariums: AANS, Medtronic.
James D. Guest: Grant support, DOD, NIH-R21/RO1, Scientific advisory board In Vivo therapeutics, consultant to Abbvie.
Mary Bartlett Bunge, W. Dalton Dietrich, James D. Guest, Aisha Khan, Allan D. Levi and Damien Pearse have disclosed a relationship with Aceso Therapeutics that includes equity.
For the other authors, no competing financial interests exist.
Figures


Similar articles
-
Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: an interim report on safety considerations and possible outcomes.Neurosci Lett. 2008 Sep 26;443(1):46-50. doi: 10.1016/j.neulet.2008.07.041. Epub 2008 Jul 22. Neurosci Lett. 2008. PMID: 18662744 Clinical Trial.
-
Safety of Autologous Human Schwann Cell Transplantation in Subacute Thoracic Spinal Cord Injury.J Neurotrauma. 2017 Nov 1;34(21):2950-2963. doi: 10.1089/neu.2016.4895. Epub 2017 Mar 21. J Neurotrauma. 2017. PMID: 28225648 Clinical Trial.
-
Imaging characteristics of chronic spinal cord injury identified during screening for a cell transplantation clinical trial.Neurosurg Focus. 2019 Mar 1;46(3):E8. doi: 10.3171/2018.12.FOCUS18593. Neurosurg Focus. 2019. PMID: 30835682
-
Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury.Curr Opin Organ Transplant. 2013 Dec;18(6):682-9. doi: 10.1097/MOT.0000000000000026. Curr Opin Organ Transplant. 2013. PMID: 24220051 Free PMC article. Review.
-
Schwann cell transplantation for repair of the adult spinal cord.J Neurotrauma. 2006 Mar-Apr;23(3-4):453-67. doi: 10.1089/neu.2006.23.453. J Neurotrauma. 2006. PMID: 16629629 Review.
Cited by
-
Stem Cell Therapy for Spinal Cord Injury: A Review of Recent Clinical Trials.Cureus. 2022 Apr 28;14(4):e24575. doi: 10.7759/cureus.24575. eCollection 2022 Apr. Cureus. 2022. PMID: 35664388 Free PMC article. Review.
-
Human Schwann Cell Transplantation for Spinal Cord Injury: Prospects and Challenges in Translational Medicine.Front Cell Neurosci. 2021 Jun 18;15:690894. doi: 10.3389/fncel.2021.690894. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34220455 Free PMC article. Review.
-
Human Schwann Cell-Derived Extracellular Vesicle Isolation, Bioactivity Assessment, and Omics Characterization.Int J Nanomedicine. 2025 Apr 4;20:4123-4144. doi: 10.2147/IJN.S500159. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40201152 Free PMC article.
-
Next-gen spinal cord injury clinical trials: lessons learned and opportunities for future success.EBioMedicine. 2024 Nov;109:105381. doi: 10.1016/j.ebiom.2024.105381. Epub 2024 Oct 8. EBioMedicine. 2024. PMID: 39383609 Free PMC article.
-
A Multi-Stage Bioprocess for the Expansion of Rodent Skin-Derived Schwann Cells in Computer-Controlled Bioreactors.Int J Mol Sci. 2023 Mar 8;24(6):5152. doi: 10.3390/ijms24065152. Int J Mol Sci. 2023. PMID: 36982227 Free PMC article.
References
-
- Bunge, R.P., Puckett, W.R., Becerra, J.L., Marcillo, A., and Quencer, R.M. (1993). Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 59, 75–89. - PubMed
-
- Cohen-Adad, J., El Mendili, M.-M., Lehéricy, S., Pradat, P.F., Blancho, S., Rossignol, S., and Benali, H. (2011). Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. NeuroImage 55, 1024–1033. - PubMed
-
- Guest, J.D., Hiester, E.D., and Bunge, R.P. (2005). Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol. 192, 384–393. - PubMed
-
- Norenberg, M.D., Smith, J., and Marcillo, A. (2004). The pathology of human spinal cord injury: defining the problems. J. Neurotrauma 21, 429–440. - PubMed
-
- Wolfe, D.L., Hayes, K.C., Hsieh, J.T., and Potter, P.J. (2001). Effects of 4-aminopyridine on motor evoked potentials in patients with spinal cord injury: a double-blinded, placebo-controlled crossover trial. J. Neurotrauma 18, 757–771. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

