Alpha-actinin-4 is essential for maintaining normal trophoblast proliferation and differentiation during early pregnancy
- PMID: 33757527
- PMCID: PMC7986381
- DOI: 10.1186/s12958-021-00733-0
Alpha-actinin-4 is essential for maintaining normal trophoblast proliferation and differentiation during early pregnancy
Abstract
Background: Proper differentiation of trophoblasts in the human placenta is essential for a successful pregnancy, whereas abnormal regulation of this process may lead to adverse pregnancy outcomes, especially preeclampsia (PE). However, the underlying mechanism of trophoblast differentiation remains unclear. Previous studies have reported the involvement of alpha-actinin-4 (ACTN4) in the actin cytoskeleton dynamics and motility. Hence, we hypothesized that ACTN4 may act as an important regulator in the normal proliferation and differentiation of trophoblasts during early pregnancy.
Method: To test this hypothesis, we collected villous tissues from women undergoing a legal pregnancy termination during 6-10 weeks of gestation and explanted them for cell culture and siRNA transfection. We also obtained placental tissues from PE patients and healthy pregnant women and isolated the primary cytotrophoblast (CTB) cells. The expression of ACTN4 in the CTBs of placental villi and during the differentiation of CTBs into STBs was detected by immunofluorescence, immunohistochemistry (IHC), and EdU proliferation assays. Besides, villous explant, Matrigel invasion, transwell migration assay, and Wound-healing assay were performed to identify the possible role of ACTN4 in the outgrowth of explants and the invasion, migration, and proliferation of cell column trophoblasts (CCTs). Western blot analysis was carried out to compare the protein expression level of AKT, Snail activities, and epithelial-to-mesenchymal transition (EMT) in the villi or HTR8/SVneo cells with ACTN4 knockdown.
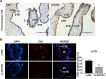
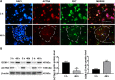
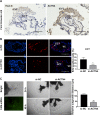
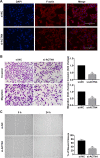
Results: ACTN4 was highly expressed in CTB cells and interstitial extravillous trophoblast (iEVT) cells but not found in the syncytiotrophoblast (STB) cells in the first trimester villi. Downregulation of ACTN4 led to reduced trophoblast proliferation and explant outgrowth ex vivo, as well as iEVT invasion and migration in vitro due to disrupt of actin filaments organization. Such ACTN4 inhibition also decreased AKT and Snail activities and further impeded the EMT process. In addition, ACTN4 expression was found to be downregulated in the iEVTs from preeclamptic placentas.
Conclusions: Our findings suggest that ACTN4 may act as an important regulator of trophoblast proliferation and differentiation during early pregnancy, and dysregulation of this protein may contribute to preeclampsia pathogenesis.
Keywords: ACTN4; Invasion and migration; Preeclampsia; Proliferation; Trophoblast.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

Similar articles
-
Trophoblastic proliferation and invasion regulated by ACTN4 is impaired in early onset preeclampsia.FASEB J. 2019 May;33(5):6327-6338. doi: 10.1096/fj.201802058RR. Epub 2019 Feb 18. FASEB J. 2019. PMID: 30776251
-
Downregulated N-acetylglucosaminyltransferase III is involved in attenuating trophoblast migration and invasion under hypoxia-reoxygenation condition.J Matern Fetal Neonatal Med. 2019 Jul;32(14):2369-2375. doi: 10.1080/14767058.2018.1438392. Epub 2018 Feb 21. J Matern Fetal Neonatal Med. 2019. PMID: 29466889
-
Expression of Gadd45α in human early placenta and its role in trophoblast invasion.Placenta. 2014 Jun;35(6):370-7. doi: 10.1016/j.placenta.2014.03.020. Epub 2014 Apr 12. Placenta. 2014. PMID: 24755561
-
WNT and NOTCH signaling in human trophoblast development and differentiation.Cell Mol Life Sci. 2022 May 13;79(6):292. doi: 10.1007/s00018-022-04285-3. Cell Mol Life Sci. 2022. PMID: 35562545 Free PMC article. Review.
-
Trophoblast invasion: Lessons from abnormally invasive placenta (placenta accreta).Placenta. 2020 Dec;102:61-66. doi: 10.1016/j.placenta.2020.01.004. Epub 2020 Jan 10. Placenta. 2020. PMID: 33218581 Free PMC article. Review.
Cited by
-
Associations Between Prenatal Vitamin D and Placental Gene Expression.bioRxiv [Preprint]. 2024 May 12:2024.05.10.593571. doi: 10.1101/2024.05.10.593571. bioRxiv. 2024. Update in: J Nutr. 2024 Dec;154(12):3603-3614. doi: 10.1016/j.tjnut.2024.10.019. PMID: 38765981 Free PMC article. Updated. Preprint.
-
Exploring the potential role of palladin in modulating human CAF/ECM functional units.Cytoskeleton (Hoboken). 2025 Mar;82(3):175-185. doi: 10.1002/cm.21926. Epub 2024 Sep 6. Cytoskeleton (Hoboken). 2025. PMID: 39239855 Review.
-
Associations Between Prenatal Vitamin D and Placental Gene Expression.J Nutr. 2024 Dec;154(12):3603-3614. doi: 10.1016/j.tjnut.2024.10.019. Epub 2024 Oct 12. J Nutr. 2024. PMID: 39401684 Free PMC article.
-
Exosome-delivered miR-410-3p reverses epithelial-mesenchymal transition, migration and invasion of trophoblasts in spontaneous abortion.J Cell Mol Med. 2024 Feb;28(3):e18097. doi: 10.1111/jcmm.18097. Epub 2024 Jan 2. J Cell Mol Med. 2024. PMID: 38164738 Free PMC article.
-
Proteomics Analysis of Porcine Endometrial Cell-Derived Extracellular Vesicles Involved in Embryo Attachment.Mol Cell Proteomics. 2025 Apr;24(4):100942. doi: 10.1016/j.mcpro.2025.100942. Epub 2025 Mar 11. Mol Cell Proteomics. 2025. PMID: 40081538 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

