Urinary metabolites predict mortality or need for renal replacement therapy after combat injury
- PMID: 33757577
- PMCID: PMC7988986
- DOI: 10.1186/s13054-021-03544-2
Urinary metabolites predict mortality or need for renal replacement therapy after combat injury
Abstract
Background: Traditionally, patient risk scoring is done by evaluating vital signs and clinical severity scores with clinical intuition. Urinary biomarkers can add objectivity to these models to make risk prediction more accurate. We used metabolomics to identify prognostic urinary biomarkers of mortality or need for renal replacement therapy (RRT). Additionally, we assessed acute kidney injury (AKI) diagnosis, injury severity score (ISS), and AKI stage.
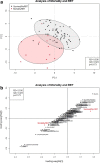
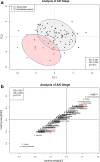
Methods: Urine samples (n = 82) from a previous study of combat casualties were evaluated using proton nuclear magnetic resonance (1H-NMR) spectroscopy. Chenomx software was used to identify and quantify urinary metabolites. Metabolite concentrations were normalized by urine output, autoscaled, and log-transformed. Partial least squares discriminant analysis (PLS-DA) and statistical analysis were performed. Receiver operating characteristic (ROC) curves were used to assess prognostic utility of biomarkers for mortality and RRT.
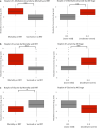
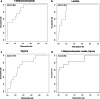
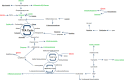
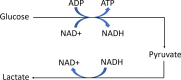
Results: Eighty-four (84) metabolites were identified and quantified in each urine sample. Of these, 11 were identified as drugs or drug metabolites and excluded. The PLS-DA models for ISS and AKI diagnosis did not have acceptable model statistics. Therefore, only mortality/RRT and AKI stage were analyzed further. Of 73 analyzed metabolites, 9 were significantly associated with mortality/RRT (p < 0.05) and 11 were significantly associated with AKI stage (p < 0.05). 1-Methylnicotinamide was the only metabolite to be significantly associated (p < 0.05) with all outcomes and was significantly higher (p < 0.05) in patients with adverse outcomes. Elevated lactate and 1-methylnicotinamide levels were associated with higher AKI stage and mortality and RRT, whereas elevated glycine levels were associated with patients who survived and did not require RRT, or had less severe AKI. ROC curves for each of these metabolites and the combined panel had good predictive value (lactate AUC = 0.901, 1-methylnicotinamide AUC = 0.864, glycine AUC = 0.735, panel AUC = 0.858).
Conclusions: We identified urinary metabolites associated with AKI stage and the primary outcome of mortality or need for RRT. Lactate, 1-methylnicotinamide, and glycine may be used as a panel of predictive biomarkers for mortality and RRT. 1-Methylnicotinamide is a novel biomarker associated with adverse outcomes. Additional studies are necessary to determine how these metabolites can be utilized in clinically-relevant risk prediction models.
Keywords: Acute kidney injury; Biomarkers; Combat injury; Metabolites; Metabolomics; Renal replacement therapy; Risk prediction.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Biomarker Predictors of Adverse Acute Kidney Injury Outcomes in Critically Ill Patients: The Dublin Acute Biomarker Group Evaluation Study.Am J Nephrol. 2019;50(1):19-28. doi: 10.1159/000500231. Epub 2019 Jun 14. Am J Nephrol. 2019. PMID: 31203271
-
Prediction of the renal replacement therapy requirement in mechanically ventilated critically ill patients by combining biomarkers for glomerular filtration and tubular damage.J Crit Care. 2014 Aug;29(4):692.e7-13. doi: 10.1016/j.jcrc.2014.02.011. Epub 2014 Feb 25. J Crit Care. 2014. PMID: 24674762
-
Urinary calprotectin, kidney injury molecule-1, and neutrophil gelatinase-associated lipocalin for the prediction of adverse outcome in pediatric acute kidney injury.Eur J Pediatr. 2017 Jun;176(6):745-755. doi: 10.1007/s00431-017-2907-y. Epub 2017 Apr 14. Eur J Pediatr. 2017. PMID: 28409285 Clinical Trial.
-
Novel biomarkers for predicting successful liberation of renal replacement therapy for acute kidney injury: a systematic review.Crit Care. 2025 May 26;29(1):213. doi: 10.1186/s13054-025-05451-2. Crit Care. 2025. PMID: 40420198 Free PMC article.
-
Furosemide stress test as a predictive marker of acute kidney injury progression or renal replacement therapy: a systemic review and meta-analysis.Crit Care. 2020 May 7;24(1):202. doi: 10.1186/s13054-020-02912-8. Crit Care. 2020. PMID: 32381019 Free PMC article.
Cited by
-
Probiotics in septic acute kidney injury, a double blind, randomized control trial.Ren Fail. 2023;45(2):2260003. doi: 10.1080/0886022X.2023.2260003. Epub 2023 Sep 19. Ren Fail. 2023. PMID: 37724527 Free PMC article. Clinical Trial.
-
Nicotinamide Adenine Dinucleotide Biosynthetic Impairment and Urinary Metabolomic Alterations Observed in Hospitalized Adults With COVID-19-Related Acute Kidney Injury.Kidney Int Rep. 2021 Dec;6(12):3002-3013. doi: 10.1016/j.ekir.2021.09.001. Epub 2021 Sep 14. Kidney Int Rep. 2021. PMID: 34541422 Free PMC article.
-
Metabolomics for the Identification of Biomarkers in Kidney Diseases.Nanotheranostics. 2025 Mar 24;9(2):110-120. doi: 10.7150/ntno.108320. eCollection 2025. Nanotheranostics. 2025. PMID: 40212952 Free PMC article. Review.
-
Hydroxysafflor Yellow A modulation of metabolite networks and inhibition of JAK2/STAT1 pathway in sepsis.Sci Rep. 2025 Jul 16;15(1):25861. doi: 10.1038/s41598-025-11545-2. Sci Rep. 2025. PMID: 40670549 Free PMC article.
-
Metabolomics- and proteomics-based multi-omics integration reveals early metabolite alterations in sepsis-associated acute kidney injury.BMC Med. 2025 Feb 11;23(1):79. doi: 10.1186/s12916-025-03920-7. BMC Med. 2025. PMID: 39934788 Free PMC article.
References
-
- Stewart IJ, Glass KR, Howard JT, Morrow BD, Sosnov JA, Siew ED, Wickersham N, Latack W, Kwan HK, Heegard KD, Diaz C, Henderson AT, Saenz KK, Ikizler TA, Chung KK. The potential utility of urinary biomarkers for risk prediction in combat casualties: a prospective observational cohort study. Crit Care. 2015;19:252. doi: 10.1186/s13054-015-0965-y. - DOI - PMC - PubMed
-
- Champion HR, Bellamy RF, Roberts P, Leppaniemi AA. Profile of combat injury. J Trauma Injury Infect Crit Care. 2003;54(5):S13–S19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

