Space-occupying brain lesions, trauma-related tau astrogliopathy, and ARTAG: a report of two cases and a literature review
- PMID: 33757579
- PMCID: PMC7986305
- DOI: 10.1186/s40478-021-01152-3
Space-occupying brain lesions, trauma-related tau astrogliopathy, and ARTAG: a report of two cases and a literature review
Abstract
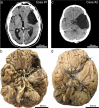
Astrocytes with intracellular accumulations of misfolded phosphorylated tau protein have been observed in advanced-stage chronic traumatic encephalopathy (CTE) and in other neurodegenerative conditions. There is a growing awareness that astrocytic tau inclusions are also relatively common in the brains of persons over 70 years of age-affecting approximately one-third of autopsied individuals. The pathologic hallmarks of aging-related tau astrogliopathy (ARTAG) include phosphorylated tau protein within thorn-shaped astrocytes (TSA) in subpial, subependymal, perivascular, and white matter regions, whereas granular-fuzzy astrocytes are often seen in gray matter. CTE and ARTAG share molecular and histopathologic characteristics, suggesting that trauma-related mechanism(s) may predispose to the development of tau astrogliopathy. There are presently few experimental systems to study the pathobiology of astrocytic-tau aggregation, but human studies have made recent progress. For example, leucotomy (also referred to as lobotomy) is associated with a localized ARTAG-like neuropathology decades after the surgical brain injury, suggesting that chronic brain injury of any type may predispose to later life ARTAG. To examine this idea in a different context, we report clinical and pathologic features of two middle-aged men who came to autopsy with large (> 6 cm in greatest dimension) arachnoid cysts that had physically displaced and injured the subjects' left temporal lobes through chronic mechanical stress. Despite the similarity of the size and location of the arachnoid cysts, these individuals had dissimilar neurologic outcomes and neuropathologic findings. We review the evidence for ARTAG in response to brain injury, and discuss how the location and molecular properties of astroglial tau inclusions might alter the physiology of resident astrocytes. These cases and literature review point toward possible mechanism(s) of tau aggregation in astrocytes in response to chronic brain trauma.
Keywords: FTLD; Meninges; NFTs; PSP; TBI; Tangles; Tauopathy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Shively SB, Edgerton SL, Iacono D, Purohit DP, Qu BX, Haroutunian V, Davis KL, Diaz-Arrastia R, Perl DP. Localized cortical chronic traumatic encephalopathy pathology after single, severe axonal injury in human brain. Acta Neuropathol. 2017;133(3):353–366. doi: 10.1007/s00401-016-1649-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

