Octanoate is differentially metabolized in liver and muscle and fails to rescue cardiomyopathy in CPT2 deficiency
- PMID: 33757734
- PMCID: PMC8082564
- DOI: 10.1016/j.jlr.2021.100069
Octanoate is differentially metabolized in liver and muscle and fails to rescue cardiomyopathy in CPT2 deficiency
Abstract
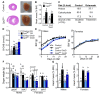
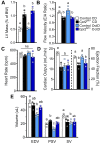
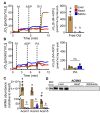
Long-chain fatty acid oxidation is frequently impaired in primary and systemic metabolic diseases affecting the heart; thus, therapeutically increasing reliance on normally minor energetic substrates, such as ketones and medium-chain fatty acids, could benefit cardiac health. However, the molecular fundamentals of this therapy are not fully known. Here, we explored the ability of octanoate, an eight-carbon medium-chain fatty acid known as an unregulated mitochondrial energetic substrate, to ameliorate cardiac hypertrophy in long-chain fatty acid oxidation-deficient hearts because of carnitine palmitoyltransferase 2 deletion (Cpt2M-/-). CPT2 converts acylcarnitines to acyl-CoAs in the mitochondrial matrix for oxidative bioenergetic metabolism. In Cpt2M-/- mice, high octanoate-ketogenic diet failed to alleviate myocardial hypertrophy, dysfunction, and acylcarnitine accumulation suggesting that this alternative substrate is not sufficiently compensatory for energy provision. Aligning this outcome, we identified a major metabolic distinction between muscles and liver, wherein heart and skeletal muscle mitochondria were unable to oxidize free octanoate, but liver was able to oxidize free octanoate. Liver mitochondria, but not heart or muscle, highly expressed medium-chain acyl-CoA synthetases, potentially enabling octanoate activation for oxidation and circumventing acylcarnitine shuttling. Conversely, octanoylcarnitine was oxidized by liver, skeletal muscle, and heart, with rates in heart 4-fold greater than liver and, in muscles, was not dependent upon CPT2. Together, these data suggest that dietary octanoate cannot rescue CPT2-deficient cardiac disease. These data also suggest the existence of tissue-specific mechanisms for octanoate oxidative metabolism, with liver being independent of free carnitine availability, whereas cardiac and skeletal muscles depend on carnitine but not on CPT2.
Keywords: carnitine palmitoyltransferase; carnitine shuttle; fatty acid oxidation; medium-chain fatty acids; mitochondria.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Ormerod J.O.M., Frenneaux M.P., Sherrid M.V. Myocardial energy depletion and dynamic systolic dysfunction in hypertrophic cardiomyopathy. Nat. Rev. Cardiol. 2016;13:677–687. - PubMed
-
- Bianco H.T., Izar M.C., Póvoa R.M., Bombig M.T., Fonseca H.A., Helfenstein T., Ferreira C.E., Nicolau J.C., Neto A.A., Feio C.M., Cerci M.S., Fonseca F.A. Left ventricular hypertrophy and QTc dispersion are predictors of long-term mortality in subjects with type 2 diabetes. Int. J. Cardiol. 2014;176:1170–1172. - PubMed
-
- Struthers A.D., Morris A.D. Screening for and treating left-ventricular abnormalities in diabetes mellitus: a new way of reducing cardiac deaths. Lancet. 2002;359:1430–1432. - PubMed
-
- Murdolo G., Angeli F., Reboldi G., Di Giacomo L., Aita A., Bartolini C., Vedecchia P. Left ventricular hypertrophy and obesity: only a matter of fat? High Blood Press. Cardiovasc. Prev. 2015;22:29–41. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

