Compartmental diffusion and microstructural properties of human brain gray and white matter studied with double diffusion encoding magnetic resonance spectroscopy of metabolites and water
- PMID: 33757904
- PMCID: PMC8204266
- DOI: 10.1016/j.neuroimage.2021.117981
Compartmental diffusion and microstructural properties of human brain gray and white matter studied with double diffusion encoding magnetic resonance spectroscopy of metabolites and water
Abstract
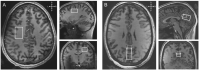
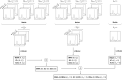
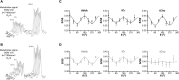
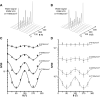
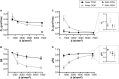
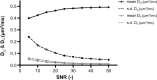
Double diffusion encoding (DDE) of the water signal offers a unique ability to separate the effect of microscopic anisotropic diffusion in structural units of tissue from the overall macroscopic orientational distribution of cells. However, the specificity in detected microscopic anisotropy is limited as the signal is averaged over different cell types and across tissue compartments. Performing side-by-side water and metabolite DDE spectroscopic (DDES) experiments provides complementary measures from which intracellular and extracellular microscopic fractional anisotropies (μFA) and diffusivities can be estimated. Metabolites are largely confined to the intracellular space and therefore provide a benchmark for intracellular μFA and diffusivities of specific cell types. By contrast, water DDES measurements allow examination of the separate contributions to water μFA and diffusivity from the intra- and extracellular spaces, by using a wide range of b values to gradually eliminate the extracellular contribution. Here, we aimed to estimate tissue and compartment specific human brain microstructure by combining water and metabolites DDES experiments. We performed our DDES measurements in two brain regions that contain widely different amounts of white matter (WM) and gray matter (GM): parietal white matter (PWM) and occipital gray matter (OGM) in a total of 20 healthy volunteers at 7 Tesla. Metabolite DDES measurements were performed at b = 7199 s/mm2, while water DDES measurements were performed with a range of b values from 918 to 7199 s/mm2. The experimental framework we employed here resulted in a set of insights pertaining to the morphology of the intracellular and extracellular spaces in both gray and white matter. Results of the metabolite DDES experiments in both PWM and OGM suggest a highly anisotropic intracellular space within neurons and glia, with the possible exception of gray matter glia. The water μFA obtained from the DDES results at high b values in both regions converged with that of the metabolite DDES, suggesting that the signal from the extracellular space is indeed effectively suppressed at the highest b value. The μFA measured in the OGM significantly decreased at lower b values, suggesting a considerably lower anisotropy of the extracellular space in GM compared to WM. In PWM, the water μFA remained high even at the lowest b value, indicating a high degree of organization in the interstitial space in WM. Tortuosity values in the cytoplasm for water and tNAA, obtained with correlation analysis of microscopic parallel diffusivity with respect to GM/WM tissue fraction in the volume of interest, are remarkably similar for both molecules, while exhibiting a clear difference between gray and white matter, suggesting a more crowded cytoplasm and more complex cytomorphology of neuronal cell bodies and dendrites in GM than those found in long-range axons in WM.
Keywords: Compartment specificity; Double diffusion encoding; Human brain tissue; Magnetic resonance spectroscopy; Microscopic anisotropy.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest None.
Figures



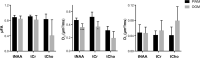


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

