Age dependent associations of risk factors with heart failure: pooled population based cohort study
- PMID: 33758001
- PMCID: PMC7986583
- DOI: 10.1136/bmj.n461
Age dependent associations of risk factors with heart failure: pooled population based cohort study
Erratum in
-
Age dependent associations of risk factors with heart failure: pooled population based cohort study.BMJ. 2021 Apr 1;373:n880. doi: 10.1136/bmj.n880. BMJ. 2021. PMID: 33795254 Free PMC article. No abstract available.
Abstract
Objective: To assess age differences in risk factors for incident heart failure in the general population.
Design: Pooled population based cohort study.
Setting: Framingham Heart Study, Prevention of Renal and Vascular End-stage Disease Study, and Multi-Ethnic Study of Atherosclerosis.
Participants: 24 675 participants without a history of heart failure stratified by age into young (<55 years; n=11 599), middle aged (55-64 years; n=5587), old (65-74 years; n=5190), and elderly (≥75 years; n=2299) individuals.
Main outcome measure: Incident heart failure.
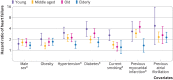
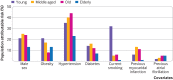
Results: Over a median follow-up of 12.7 years, 138/11 599 (1%), 293/5587 (5%), 538/5190 (10%), and 412/2299 (18%) of young, middle aged, old, and elderly participants, respectively, developed heart failure. In young participants, 32% (n=44) of heart failure cases were classified as heart failure with preserved ejection fraction compared with 43% (n=179) in elderly participants. Risk factors including hypertension, diabetes, current smoking history, and previous myocardial infarction conferred greater relative risk in younger compared with older participants (P for interaction <0.05 for all). For example, hypertension was associated with a threefold increase in risk of future heart failure in young participants (hazard ratio 3.02, 95% confidence interval 2.10 to 4.34; P<0.001) compared with a 1.4-fold risk in elderly participants (1.43, 1.13 to 1.81; P=0.003). The absolute risk for developing heart failure was lower in younger than in older participants with and without risk factors. Importantly, known risk factors explained a greater proportion of overall population attributable risk for heart failure in young participants (75% v 53% in elderly participants), with better model performance (C index 0.79 v 0.64). Similarly, the population attributable risks of obesity (21% v 13%), hypertension (35% v 23%), diabetes (14% v 7%), and current smoking (32% v 1%) were higher in young compared with elderly participants.
Conclusions: Despite a lower incidence and absolute risk of heart failure among younger compared with older people, the stronger association and greater attributable risk of modifiable risk factors among young participants highlight the importance of preventive efforts across the adult life course.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Funding: This work was partially supported by the National Heart, Lung and Blood Institute (NHLBI), including the Framingham Heart Study (contract N01-HC25195 and HHSN268201500001I). MESA and the MESA SHARe project are conducted and supported by the NHLBI in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1-TR-001881, and DK063491. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The Prevention of Renal and Vascular End-Stage Disease (PREVEND) study has been made possible by grants from the Dutch Kidney Foundation. RAB is supported by the Netherlands Heart Foundation (CVON- DOSIS, grant 2014-40; CVON SHE-PREDICTS-HF, grant 2017-021, and CVON RED CVD 2017-11). JEH is supported by NIH grants R01-HL134893, R01-HL140224, and K24-HL153669. DEL is supported by a mid-career award from the Heart and Stroke Foundation of Canada and is the Ted Rogers Chair in Heart Function Outcomes. DL’s research is supported by the Division of Intramural Research, National, Heart, Lung, and Blood Institute, National Institutes of Health. VSR is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment, Boston University School of Medicine. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the US Department of Health and Human Services. The funders had no role in the conduct of the study, collection, management, analysis and interpretation of the data, preparation, review and approval of the manuscript, or the decision to submit the manuscript for publication. Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: funding for the work as detailed above; JT has received personal fees from Roche diagnostics, Olink proteomics, and Us2.ai, outside the submitted work, and has a patent 16/216,929 licensed; MJB has received grants from NIH, FDA, AHA, and Aetna Foundation, grants and personal fees from Novo Nordisk and Amgen Foundation, and personal fees from Sanofi, Regeneron, Novartis, Bayer, 89Bio, Kaleido, Inozyme, and Kowa, outside the submitted work; SJS has received grants and personal fees from Actelion, AstraZeneca, Pfizer, and Novartis, grants from Corvia, and personal fees from Abbott, Amgen, Aria CV, Axon, Bayer, Bristol Myers Squib, Boehringer-Ingelheim, Boston Scientific, Boxer Capital, Cardiora, CVRx, Cyclerion, Cytokinetics, Edwards Lifesciences , personal fees from Eisai, eKo.ai, GSK, Imara, Ionis, Ironwood, Janssen, Keyto, Lilly Medical, Merck, MyoKardia, Novo Nordisk, Prothena, Regeneron, Sanofi, Shifamed, Tenax, and United Therapeutics, outside the submitted work; RAB has received grants from AstraZeneca, Abbott, Bristol-Myers Squibb, Novartis, Novo Nordisk, and Roche, and personal fees from Abbott, AstraZeneca, Bayer, Novartis, and Roche, outside the submitted work; CSPL has received grants from Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, and Vifor Pharma, and personal fees from Abbott Diagnostics, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Biofourmis, Boehringer Ingelheim, Boston Scientific, Corvia Medical, Cytokinetics, Darma Inc, Us2.ai, JanaCare, Janssen Research & Development LLC, Medtronic, Menarini Group, Merck, MyoKardia, Novartis, Novo Nordisk, Radcliffe Group Ltd, Roche Diagnostics, Sanofi, Stealth BioTherapeutics, The Corpus, Vifor Pharma, and WebMD Global LLC, outside the submitted work, and has a patent PCT/SG2016/050217 pending and a patent 16/216,929 issued; JEH has received grants from NIH/NHLBI, during the conduct of the study, grants from Bayer AG and Gilead Sciences, and other support from EcoNugenics Inc, outside the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
Publication types
MeSH terms
Grants and funding
- T32 HL094301/HL/NHLBI NIH HHS/United States
- N01HC95169/HL/NHLBI NIH HHS/United States
- R01 HL140224/HL/NHLBI NIH HHS/United States
- N01HC95168/HL/NHLBI NIH HHS/United States
- N01HC95162/HL/NHLBI NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- N01HC95165/HL/NHLBI NIH HHS/United States
- N01HC95167/HL/NHLBI NIH HHS/United States
- N01HC95159/HL/NHLBI NIH HHS/United States
- N01HC95163/HL/NHLBI NIH HHS/United States
- N01HC25195/HL/NHLBI NIH HHS/United States
- N01HC95164/HL/NHLBI NIH HHS/United States
- P30 DK063491/DK/NIDDK NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- N01HC95160/HL/NHLBI NIH HHS/United States
- N01HC95161/HL/NHLBI NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- UL1 TR001079/TR/NCATS NIH HHS/United States
- N01HC95166/HL/NHLBI NIH HHS/United States
- K24 HL153669/HL/NHLBI NIH HHS/United States
- R01 HL134893/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
