Hypercholesterolemia in Progressive Renal Failure Is Associated with Changes in Hepatic Heparan Sulfate - PCSK9 Interaction
- PMID: 33758009
- PMCID: PMC8259657
- DOI: 10.1681/ASN.2020091376
Hypercholesterolemia in Progressive Renal Failure Is Associated with Changes in Hepatic Heparan Sulfate - PCSK9 Interaction
Abstract
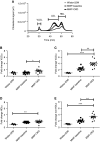
Background: Dyslipidemia is an important risk factor in CKD. The liver clears triglyceride-rich lipoproteins (TRL) via LDL receptor (LDLR), LDLR-related protein-1 (LRP-1), and heparan sulfate proteoglycans (HSPGs), mostly syndecan-1. HSPGs also facilitate LDLR degradation by proprotein convertase subtilisin/kexin type 9 (PCSK9). Progressive renal failure affects the structure and activity of hepatic lipoprotein receptors, PCSK9, and plasma cholesterol.
Methods: Uninephrectomy- and aging-induced CKD in normotensive Wistar rats and hypertensive Munich-Wistar-Frömter (MWF) rats.
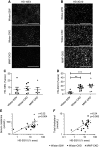
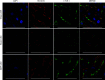
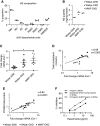
Results: Compared with 22-week-old sex- and strain-matched rats, 48-week-old uninephrectomized Wistar-CKD and MWF-CKD rats showed proteinuria, increased plasma creatinine, and hypercholesterolemia (all P<0.05), which were most apparent in hypertensive MWF-CKD rats. Hepatic PCSK9 expression increased in both CKD groups (P<0.05), with unusual sinusoidal localization, which was not seen in 22-week-old rats. Heparan sulfate (HS) disaccharide analysis, staining with anti-HS mAbs, and mRNA expression of HS polymerase exostosin-1 (Ext-1), revealed elongated HS chains in both CKD groups. Solid-phase competition assays showed that the PCSK9 interaction with heparin-albumin (HS-proteoglycan analogue) was critically dependent on polysaccharide chain length. VLDL binding to HS from CKD livers was reduced (P<0.05). Proteinuria and plasma creatinine strongly associated with plasma cholesterol, PCSK9, and HS changes.
Conclusions: Progressive CKD induces hepatic HS elongation, leading to increased interaction with PCSK9. This might reduce hepatic lipoprotein uptake and thereby induce dyslipidemia in CKD. Therefore, PCSK9/HS may be a novel target to control dyslipidemia.
Keywords: PCSK9; chronic kidney disease; dyslipidemia; heparan sulfate; syndecan-1.
Copyright © 2021 by the American Society of Nephrology.
Figures





Similar articles
-
Emerging roles of PCSK9 in kidney disease: lipid metabolism, megalin regulation and proteinuria.Pflugers Arch. 2025 Jun;477(6):773-786. doi: 10.1007/s00424-025-03069-5. Epub 2025 Feb 18. Pflugers Arch. 2025. PMID: 39964484 Review.
-
Novel aspects of PCSK9 and lipoprotein receptors in renal disease-related dyslipidemia.Cell Signal. 2019 Mar;55:53-64. doi: 10.1016/j.cellsig.2018.12.001. Epub 2018 Dec 12. Cell Signal. 2019. PMID: 30550765 Review.
-
Proteinuria converts hepatic heparan sulfate to an effective proprotein convertase subtilisin kexin type 9 enzyme binding partner.Kidney Int. 2021 Jun;99(6):1369-1381. doi: 10.1016/j.kint.2021.01.023. Epub 2021 Feb 18. Kidney Int. 2021. PMID: 33609572
-
LDL-Bound PCSK9 Has a Slower Clearance Kinetic and Higher Use for HSPGs Than Free-PCSK9-Brief Report.Arterioscler Thromb Vasc Biol. 2025 Sep;45(9):1565-1573. doi: 10.1161/ATVBAHA.124.322334. Epub 2025 Jul 3. Arterioscler Thromb Vasc Biol. 2025. PMID: 40605748
-
Heparan sulfate proteoglycans present PCSK9 to the LDL receptor.Nat Commun. 2017 Sep 11;8(1):503. doi: 10.1038/s41467-017-00568-7. Nat Commun. 2017. PMID: 28894089 Free PMC article.
Cited by
-
Emerging roles of PCSK9 in kidney disease: lipid metabolism, megalin regulation and proteinuria.Pflugers Arch. 2025 Jun;477(6):773-786. doi: 10.1007/s00424-025-03069-5. Epub 2025 Feb 18. Pflugers Arch. 2025. PMID: 39964484 Review.
-
A p21-ATD mouse model for monitoring and eliminating senescent cells and its application in liver regeneration post injury.Mol Ther. 2024 Sep 4;32(9):2992-3011. doi: 10.1016/j.ymthe.2024.04.002. Epub 2024 Apr 6. Mol Ther. 2024. PMID: 38582962
-
Prevention of Triglyceridemia by (Non-)Anticoagulant Heparin(oids) Does Not Preclude Transplant Vasculopathy and Glomerulosclerosis.Front Cell Dev Biol. 2022 Mar 7;10:798088. doi: 10.3389/fcell.2022.798088. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35345850 Free PMC article.
References
-
- Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. .: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004. - PubMed
-
- Cases A, Coll E: Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int Suppl 68: S87–S93, 2005. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

