The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia
- PMID: 33758017
- PMCID: PMC8139426
- DOI: 10.1126/science.abf9648
The impact of population-wide rapid antigen testing on SARS-CoV-2 prevalence in Slovakia
Abstract
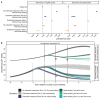
Slovakia conducted multiple rounds of population-wide rapid antigen testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in late 2020, combined with a period of additional contact restrictions. Observed prevalence decreased by 58% (95% confidence interval: 57 to 58%) within 1 week in the 45 counties that were subject to two rounds of mass testing, an estimate that remained robust when adjusting for multiple potential confounders. Adjusting for epidemic growth of 4.4% (1.1 to 6.9%) per day preceding the mass testing campaign, the estimated decrease in prevalence compared with a scenario of unmitigated growth was 70% (67 to 73%). Modeling indicated that this decrease could not be explained solely by infection control measures but required the addition of the isolation and quarantine of household members of those testing positive.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


Comment in
-
Rapid antigen testing in COVID-19 responses.Science. 2021 May 7;372(6542):571-572. doi: 10.1126/science.abi6680. Science. 2021. PMID: 33958462 No abstract available.
References
-
- Guidelines for the implementation of non-pharmaceutical interventions against COVID-19. Eur. Cent. Dis. Prev. Control (2020); www.ecdc.europa.eu/en/publications-data/covid-19-guidelines-non-pharmace....
-
- Davies N. G., et al., Lancet Public Health 10.1016/S2468-2667(20)30133-X (2020).
-
- Flaxman S., Mishra S., Gandy A., Unwin H. J. T., Mellan T. A., Coupland H., Whittaker C., Zhu H., Berah T., Eaton J. W., Monod M., Ghani A. C., Donnelly C. A., Riley S., Vollmer M. A. C., Ferguson N. M., Okell L. C., Bhatt S.; Imperial College COVID-19 Response Team , Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 584, 257–261. (2020). 10.1038/s41586-020-2405-7 - DOI - PubMed
-
- Everyone Included, Social Impact of COVID-19Dros. Inf. Serv. D; www.un.org/development/desa/dspd/everyone-included-covid-19.html.
-
- Socio-economic impact of COVID-19. UNDP; www.undp.org/content/undp/en/home/coronavirus/socio-economic-impact-of-c....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

