Essential requirement for JPT2 in NAADP-evoked Ca2+ signaling
- PMID: 33758061
- PMCID: PMC8315109
- DOI: 10.1126/scisignal.abd5605
Essential requirement for JPT2 in NAADP-evoked Ca2+ signaling
Abstract
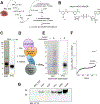
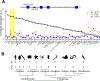
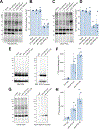
Nicotinic acid adenine dinucleotide phosphate (NAADP) is a second messenger that releases Ca2+ from acidic organelles through the activation of two-pore channels (TPCs) to regulate endolysosomal trafficking events. NAADP action is mediated by NAADP-binding protein(s) of unknown identity that confer NAADP sensitivity to TPCs. Here, we used a "clickable" NAADP-based photoprobe to isolate human NAADP-binding proteins and identified Jupiter microtubule-associated homolog 2 (JPT2) as a TPC accessory protein required for endogenous NAADP-evoked Ca2+ signaling. JPT2 was also required for the translocation of a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pseudovirus through the endolysosomal system. Thus, JPT2 is a component of the NAADP receptor complex that is essential for TPC-dependent Ca2+ signaling and control of coronaviral entry.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures




Similar articles
-
Convergent activation of two-pore channels mediated by the NAADP-binding proteins JPT2 and LSM12.Sci Signal. 2023 Aug 22;16(799):eadg0485. doi: 10.1126/scisignal.adg0485. Epub 2023 Aug 22. Sci Signal. 2023. PMID: 37607218 Free PMC article.
-
NAADP-binding proteins find their identity.Trends Biochem Sci. 2022 Mar;47(3):235-249. doi: 10.1016/j.tibs.2021.10.008. Epub 2021 Nov 20. Trends Biochem Sci. 2022. PMID: 34810081 Free PMC article. Review.
-
Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells.J Biol Chem. 2012 Jan 20;287(4):2296-307. doi: 10.1074/jbc.M111.305813. Epub 2011 Nov 23. J Biol Chem. 2012. PMID: 22117075 Free PMC article.
-
Diversity of two-pore channels and the accessory NAADP receptors in intracellular Ca2+ signaling.Cell Calcium. 2022 Jun;104:102594. doi: 10.1016/j.ceca.2022.102594. Epub 2022 May 5. Cell Calcium. 2022. PMID: 35561646 Free PMC article. Review.
-
NAADP Signaling: New Kids on the Block.Cells. 2022 Mar 21;11(6):1054. doi: 10.3390/cells11061054. Cells. 2022. PMID: 35326505 Free PMC article.
Cited by
-
Structural biology of cation channels important for lysosomal calcium release.Cell Calcium. 2022 Jan;101:102519. doi: 10.1016/j.ceca.2021.102519. Epub 2021 Dec 14. Cell Calcium. 2022. PMID: 34952412 Free PMC article. Review.
-
NAADP receptors: A one-two.Cell Calcium. 2021 Dec;100:102478. doi: 10.1016/j.ceca.2021.102478. Epub 2021 Sep 20. Cell Calcium. 2021. PMID: 34600271 Free PMC article. No abstract available.
-
A plastid two-pore channel essential for inter-organelle communication and growth of Toxoplasma gondii.Nat Commun. 2021 Oct 4;12(1):5802. doi: 10.1038/s41467-021-25987-5. Nat Commun. 2021. PMID: 34608145 Free PMC article.
-
Measuring NAADP-Evoked Ca2+ Release in Permeabilized T Cells.Methods Mol Biol. 2025;2904:67-77. doi: 10.1007/978-1-0716-4414-0_5. Methods Mol Biol. 2025. PMID: 40220226
-
Public health emergency accelerated research response-the Clinical and Translational Science Institute of Southeast Wisconsin COVID-19 research initiative.Front Public Health. 2025 May 9;13:1529121. doi: 10.3389/fpubh.2025.1529121. eCollection 2025. Front Public Health. 2025. PMID: 40416685 Free PMC article.
References
-
- Galione A, A primer of NAADP-mediated Ca2+ signalling: from sea urchin eggs to mammalian cells. Cell Calcium 58, 27–47 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

