The Lst Sialyltransferase of Neisseria gonorrhoeae Can Transfer Keto-Deoxyoctanoate as the Terminal Sugar of Lipooligosaccharide: a Glyco-Achilles Heel That Provides a New Strategy for Vaccines to Prevent Gonorrhea
- PMID: 33758087
- PMCID: PMC8092323
- DOI: 10.1128/mBio.03666-20
The Lst Sialyltransferase of Neisseria gonorrhoeae Can Transfer Keto-Deoxyoctanoate as the Terminal Sugar of Lipooligosaccharide: a Glyco-Achilles Heel That Provides a New Strategy for Vaccines to Prevent Gonorrhea
Abstract
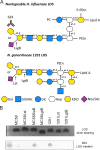
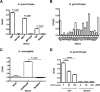
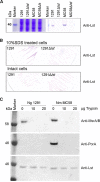
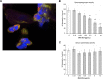
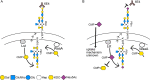
The lipooligosaccharide (LOS) of Neisseria gonorrhoeae plays key roles in pathogenesis and is composed of multiple possible glycoforms. These glycoforms are generated by the process of phase variation and by differences in the glycosyltransferase gene content of particular strains. LOS glycoforms of N. gonorrhoeae can be terminated with an N-acetylneuraminic acid (Neu5Ac), which imparts resistance to the bactericidal activity of serum. However, N. gonorrhoeae cannot synthesize the CMP-Neu5Ac required for LOS biosynthesis and must acquire it from the host. In contrast, Neisseria meningitidis can synthesize endogenous CMP-Neu5Ac, the donor molecule for Neu5Ac, which is a component of some meningococcal capsule structures. Both species have an almost identical LOS sialyltransferase, Lst, that transfers Neu5Ac from CMP-Neu5Ac to the terminus of LOS. Lst is homologous to the LsgB sialyltransferase of nontypeable Haemophilus influenzae (NTHi). Studies in NTHi have demonstrated that LsgB can transfer keto-deoxyoctanoate (KDO) from CMP-KDO to the terminus of LOS in place of Neu5Ac. Here, we show that Lst can also transfer KDO to LOS in place of Neu5Ac in both N. gonorrhoeae and N. meningitidis Consistent with access to the pool of CMP-KDO in the cytoplasm, we present data indicating that Lst is localized in the cytoplasm. Lst has previously been reported to be localized on the outer membrane. We also demonstrate that KDO is expressed as a terminal LOS structure in vivo in samples from infected women and further show that the anti-KDO monoclonal antibody 6E4 can mediate opsonophagocytic killing of N. gonorrhoeae Taken together, these studies indicate that KDO expressed on gonococcal LOS represents a new antigen for the development of vaccines against gonorrhea.IMPORTANCE The emergence of multidrug-resistant N. gonorrhoeae strains that are resistant to available antimicrobials is a current health emergency, and no vaccine is available to prevent gonococcal infection. Lipooligosaccharide (LOS) is one of the major virulence factors of N. gonorrhoeae The sialic acid N-acetylneuraminic acid (Neu5Ac) is present as the terminal glycan on LOS in N. gonorrhoeae In this study, we made an unexpected discovery that KDO can be incorporated as the terminal glycan on LOS of N. gonorrhoeae by the alpha-2,3-sialyltransferase Lst. We showed that N. gonorrhoeae express KDO on LOS in vivo and that the KDO-specific monoclonal antibody 6E4 can direct opsonophagocytic killing of N. gonorrhoeae These data support further development of KDO-LOS structures as vaccine antigens for the prevention of infection by N. gonorrhoeae.
Keywords: Lst sialyltransferase; N-acetylneuraminic acid (Neu5Ac); Neisseria gonorrhea; gonococcal vaccine; keto-deoxyoctanoate (KDO); lipooligosaccharide (LOS); sialic acid.
Copyright © 2021 Jen et al.
Figures
References
-
- Eschenbach DA. 1984. Acute pelvic inflammatory disease. Urol Clin North Am 11:65–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

