Compartmentalization of Melanin Biosynthetic Enzymes Contributes to Self-Defense against Intermediate Compound Scytalone in Botrytis cinerea
- PMID: 33758088
- PMCID: PMC8092192
- DOI: 10.1128/mBio.00007-21
Compartmentalization of Melanin Biosynthetic Enzymes Contributes to Self-Defense against Intermediate Compound Scytalone in Botrytis cinerea
Abstract
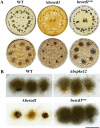
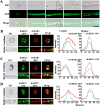
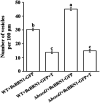
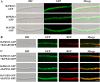
In filamentous fungi, 1,8-dihydroxynaphthalene (DHN) melanin is a major component of the extracellular matrix, endowing fungi with environmental tolerance and some pathogenic species with pathogenicity. However, the subcellular location of the melanin biosynthesis pathway components remains obscure. Using the gray mold pathogen Botrytis cinerea, the DHN melanin intermediate scytalone was characterized via phenotypic and chemical analysis of mutants, and the key enzymes participating in melanin synthesis were fused with fluorescent proteins to observe their subcellular localizations. The Δbcscd1 mutant accumulated scytalone in the culture filtrate rather than in mycelium. Excessive scytalone appears to be self-inhibitory to the fungus, leading to repressed sclerotial germination and sporulation in the Δbcscd1 mutant. The BcBRN1/2 enzymes responsible for synthesizing scytalone were localized in endosomes and found to be trafficked to the cell surface, accompanied by the accumulation of BcSCD1 proteins in the cell wall. In contrast, the early-stage melanin synthesis enzymes BcPKS12/13 and BcYGH1 were localized in peroxisomes. Taken together, the results of this study revealed the subcellular distribution of melanin biosynthetic enzymes in B. cinerea, indicating that the encapsulation and externalization of the melanin synthetic enzymes need to be delicately orchestrated to ensure enzymatic efficiency and protect itself from the adverse effect of the toxic intermediate metabolite.IMPORTANCE The devastating gray mold pathogen Botrytis cinerea propagates via melanized conidia and sclerotia. This study reveals that the sclerotial germination of B. cinerea is differentially affected by different enzymes in the melanin synthesis pathway. Using gene knockout mutants and chemical analysis, we found that excessive accumulation of the melanin intermediate scytalone is inhibitory to B. cinerea. Subcellular localization analysis of the melanin synthesis enzymes of B. cinerea suggested two-stage partitioning of the melanogenesis pathway: the intracellular stage involves the steps until the intermediate scytalone was translocated to the cell surface, whereas the extracellular stage comprises all the steps occurring in the wall from scytalone to final melanin formation. These strategies make the fungus avert self-poisoning during melanin production. This study opens avenues for better understanding the mechanisms of secondary metabolite production in filamentous fungi.
Keywords: Botrytis cinerea; DHN melanin; endosome; peroxisome; scytalone; subcellular trafficking.
Copyright © 2021 Chen et al.
Figures







References
-
- Fillinger S, Walker A. 2016. Chemical control and resistance management of Botrytis diseases, p 189–216. In Fillinger S, Elad Y (ed), Botrytis—the fungus, the pathogen and its management in agricultural systems. Springer International Publishing, Cham, Switzerland.
-
- Leroux P. 2007. Chemical control of Botrytis and its resistance to chemical fungicides, p 195–222. In Elad Y, Williamson B, Tudzynski P, Delen N (ed), Botrytis: biology, pathology and control. Springer Netherlands, Dordrecht, Netherlands.
-
- Petrasch S, Silva CJ, Mesquida-Pesci SD, Gallegos K, van den Abeele C, Papin V, Fernandez-Acero FJ, Knapp SJ, Blanco-Ulate B. 2019. Infection strategies deployed by Botrytis cinerea, Fusarium acuminatum, and Rhizopus stolonifer as a function of tomato fruit ripening stage. Front Plant Sci 10:223. doi: 10.3389/fpls.2019.00223. - DOI - PMC - PubMed
-
- Nakajima M, Akutsu K. 2014. Virulence factors of Botrytis cinerea. J Gen Plant Pathol 80:15–23. doi: 10.1007/s10327-013-0492-0. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
