Noncanonical Transmission of a Measles Virus Vaccine Strain from Neurons to Astrocytes
- PMID: 33758092
- PMCID: PMC8092232
- DOI: 10.1128/mBio.00288-21
Noncanonical Transmission of a Measles Virus Vaccine Strain from Neurons to Astrocytes
Abstract
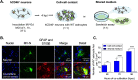
Viruses, including members of the herpes-, entero-, and morbillivirus families, are the most common cause of infectious encephalitis in mammals worldwide. During most instances of acute viral encephalitis, neurons are typically the initial cell type that is infected. However, as replication and spread ensue, other parenchymal cells can become viral targets, especially in chronic infections. Consequently, to ascertain how neurotropic viruses trigger neuropathology, it is crucial to identify which central nervous system (CNS) cell populations are susceptible and permissive throughout the course of infection, and to define how viruses spread between distinct cell types. Using a measles virus (MV) transgenic mouse model that expresses human CD46 (hCD46), the MV vaccine strain receptor, under the control of a neuron-specific enolase promoter (NSE-hCD46+ mice), a novel mode of viral spread between neurons and astrocytes was identified. Although hCD46 is required for initial neuronal infection, it is dispensable for heterotypic spread to astrocytes, which instead depends on glutamate transporters and direct neuron-astrocyte contact. Moreover, in the presence of RNase A, astrocyte infection is reduced, suggesting that nonenveloped ribonucleoproteins (RNP) may cross the neuron-astrocyte synaptic cleft. The characterization of this novel mode of intercellular transport offers insights into the unique interaction of neurons and glia and may reveal therapeutic targets to mitigate the life-threatening consequences of measles encephalitis.IMPORTANCE Viruses are the most important cause of infectious encephalitis in mammals worldwide; several thousand people, primarily the very young and the elderly, are impacted annually, and few therapies are reliably successful once neuroinvasion has occurred. To understand how viruses contribute to neuropathology, and to develop tools to prevent or ameliorate such infections, it is crucial to define if and how viruses disseminate among the different cell populations within the highly complex central nervous system. This study defines a noncanonical mode of viral transmission between neurons and astrocytes within the brain.
Keywords: astrocyte; measles virus; neuron; neurotransmitter transporters; ribonucleoprotein; synapse.
Copyright © 2021 Poelaert et al.
Figures


Similar articles
-
Interferon gamma protects neonatal neural stem/progenitor cells during measles virus infection of the brain.J Neuroinflammation. 2016 May 13;13(1):107. doi: 10.1186/s12974-016-0571-1. J Neuroinflammation. 2016. PMID: 27178303 Free PMC article.
-
Productive measles virus brain infection and apoptosis in CD46 transgenic mice.J Virol. 2000 Feb;74(3):1373-82. doi: 10.1128/jvi.74.3.1373-1382.2000. J Virol. 2000. PMID: 10627548 Free PMC article.
-
Measles virus spread between neurons requires cell contact but not CD46 expression, syncytium formation, or extracellular virus production.J Virol. 2000 Feb;74(4):1908-18. doi: 10.1128/jvi.74.4.1908-1918.2000. J Virol. 2000. PMID: 10644364 Free PMC article.
-
Making it to the synapse: measles virus spread in and among neurons.Curr Top Microbiol Immunol. 2009;330:3-30. doi: 10.1007/978-3-540-70617-5_1. Curr Top Microbiol Immunol. 2009. PMID: 19203102 Free PMC article. Review.
-
Measles virus and CD46.Curr Top Microbiol Immunol. 2009;329:31-57. doi: 10.1007/978-3-540-70523-9_3. Curr Top Microbiol Immunol. 2009. PMID: 19198561 Review.
Cited by
-
Understanding neuroinflammation through central nervous system infections.Curr Opin Neurobiol. 2022 Oct;76:102619. doi: 10.1016/j.conb.2022.102619. Epub 2022 Aug 16. Curr Opin Neurobiol. 2022. PMID: 35985075 Free PMC article. Review.
-
Why does viral RNA sometimes persist after recovery from acute infections?PLoS Biol. 2022 Jun 1;20(6):e3001687. doi: 10.1371/journal.pbio.3001687. eCollection 2022 Jun. PLoS Biol. 2022. PMID: 35648781 Free PMC article.
-
Ex vivo study of neuroinvasive and neurotropic viruses: what is current and what is next.FEMS Microbiol Rev. 2025 Jan 14;49:fuaf024. doi: 10.1093/femsre/fuaf024. FEMS Microbiol Rev. 2025. PMID: 40498321 Free PMC article. Review.
-
Multiple Receptors Involved in Invasion and Neuropathogenicity of Canine Distemper Virus: A Review.Viruses. 2022 Jul 12;14(7):1520. doi: 10.3390/v14071520. Viruses. 2022. PMID: 35891500 Free PMC article. Review.
-
The measles virus matrix F50S mutation from a lethal case of subacute sclerosing panencephalitis promotes receptor-independent neuronal spread.J Virol. 2025 Jan 31;99(1):e0175024. doi: 10.1128/jvi.01750-24. Epub 2024 Dec 6. J Virol. 2025. PMID: 39641619 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

