Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis
- PMID: 33758095
- PMCID: PMC8020656
- DOI: 10.1073/pnas.2001611118
Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis
Abstract
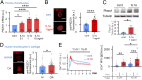
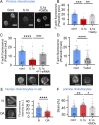
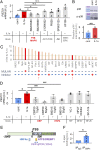
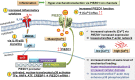
Osteoarthritis (OA) is a painful and debilitating condition of synovial joints without any disease-modifying therapies [A. M. Valdes, T. D. Spector, Nat. Rev. Rheumatol. 7, 23-32 (2011)]. We previously identified mechanosensitive PIEZO channels, PIEZO1 and PIEZO2, both expressed in articular cartilage, to function in chondrocyte mechanotransduction in response to injury [W. Lee et al., Proc. Natl. Acad. Sci. U.S.A. 111, E5114-E5122 (2014); W. Lee, F. Guilak, W. Liedtke, Curr. Top. Membr. 79, 263-273 (2017)]. We therefore asked whether interleukin-1-mediated inflammatory signaling, as occurs in OA, influences Piezo gene expression and channel function, thus indicative of maladaptive reprogramming that can be rationally targeted. Primary porcine chondrocyte culture and human osteoarthritic cartilage tissue were studied. We found that interleukin-1α (IL-1α) up-regulated Piezo1 in porcine chondrocytes. Piezo1 expression was significantly increased in human osteoarthritic cartilage. Increased Piezo1 expression in chondrocytes resulted in a feed-forward pathomechanism whereby increased function of Piezo1 induced excess intracellular Ca2+ at baseline and in response to mechanical deformation. Elevated resting state Ca2+ in turn rarefied the F-actin cytoskeleton and amplified mechanically induced deformation microtrauma. As intracellular substrates of this OA-related inflammatory pathomechanism, in porcine articular chondrocytes exposed to IL-1α, we discovered that enhanced Piezo1 expression depended on p38 MAP-kinase and transcription factors HNF4 and ATF2/CREBP1. CREBP1 directly bound to the proximal PIEZO1 gene promoter. Taken together, these signaling and genetic reprogramming events represent a detrimental Ca2+-driven feed-forward mechanism that can be rationally targeted to stem the progression of OA.
Keywords: PIEZO1; Piezo1 gene regulation; interleukin-1; osteoarthritis.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

Comment in
-
Targeting calcium-related mechanotransduction in early OA.Nat Rev Rheumatol. 2021 Aug;17(8):445-446. doi: 10.1038/s41584-021-00649-4. Nat Rev Rheumatol. 2021. PMID: 34163062 No abstract available.
References
-
- Van Spil W. E., Kubassova O., Boesen M., Bay-Jensen A. C., Mobasheri A., Osteoarthritis phenotypes and novel therapeutic targets. Biochem. Pharmacol. 165, 41–48 (2019). - PubMed
-
- Karsdal M. A., et al. ., Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: Lessons learned from failures and opportunities for the future. Osteoarthritis Cartilage 24, 2013–2021 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

