PINK1 deficiency impairs adult neurogenesis of dopaminergic neurons
- PMID: 33758225
- PMCID: PMC7988014
- DOI: 10.1038/s41598-021-84278-7
PINK1 deficiency impairs adult neurogenesis of dopaminergic neurons
Abstract
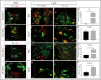
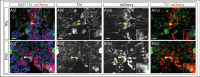
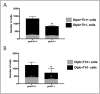
Recent evidence suggests neurogenesis is on-going throughout life but the relevance of these findings for neurodegenerative disorders such as Parkinson's disease (PD) is poorly understood. Biallelic PINK1 mutations cause early onset, Mendelian inherited PD. We studied the effect of PINK1 deficiency on adult neurogenesis of dopaminergic (DA) neurons in two complementary model systems. Zebrafish are a widely-used model to study neurogenesis in development and through adulthood. Using EdU analyses and lineage-tracing studies, we first demonstrate that a subset of ascending DA neurons and adjacent local-projecting DA neurons are each generated into adulthood in wild type zebrafish at a rate that decreases with age. Pink1-deficiency impedes DA neurogenesis in these populations, most significantly in early adult life. Pink1 already exerts an early effect on Th1+ progenitor cells rather than on differentiated DA neurons only. In addition, we investigate the effect of PINK1 deficiency in a human isogenic organoid model. Global neuronal differentiation in PINK1-deficient organoids and isogenic controls is similar, but PINK1-deficient organoids display impeded DA neurogenesis. The observation of impaired adult dopaminergic neurogenesis in Pink1 deficiency in two complementing model systems may have significant consequences for future therapeutic approaches in human PD patients with biallelic PINK1 mutations.
Conflict of interest statement
JJ and JCS are co-founders and shareholders of the biotech company OrganoTherapeutics. No other conflicts of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

