Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials
- PMID: 33758226
- PMCID: PMC7987994
- DOI: 10.1038/s41598-021-85019-6
Test conditions can significantly affect the results of in vitro cytotoxicity testing of degradable metallic biomaterials
Abstract
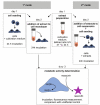
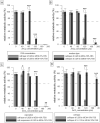
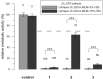
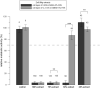
In vitro cytotoxicity testing is an indispensable part of the development of new biomaterials. However, the standard ISO 10993-5 enables variability in the testing conditions, which makes the results of the test incomparable. We studied the influence of media composition on the results of the cytotoxicity test. Solutions of ZnCl2 served as simulated extracts and we also used extracts of three types of Zn-based and Mg-based degradable metals. We incubated the cells with the solutions prepared in two types of media with two concentrations of serum (5 and 10%). We compared the toxic effect of the extracts on L929 murine fibroblast-derived cell line, which is recommended by ISO standard and on "osteoblast-like cells" U-2 OS. We also compared two methods of exposition: solutions were added either to a sub-confluent layer or to the cell suspension. We evaluated the metabolic activity of the cells using the resazurin test. We found out that in vitro cytotoxicity is dramatically influenced by the concentration of serum and by the type of the medium as well as by the type of exposition and type of cells. Therefore, when performing in vitro cytotoxicity testing of biomaterials, the authors should carefully specify the conditions of the test and comparison of different studies should be carried out with caution.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Wolf MF, Coleman KP, Lewerenz GM. Chapter II. 3.3—In vitro assessment of cell and tissue compatibility A2—Ratner, Buddy D. In: Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science. 3. Cambridge: Academic Press; 2013. pp. 593–608.
-
- J.M. Anderson, R.W. Bianco, J. Grehan, F., B.C. Grubbs, S.R. Hanson, K.D. Hauch, M. Lahi, J.P. Mrachek, S. Northup, J., Biological Testing of biomaterials, in: B.D. Ratner, A.S. Hoffman, F.J. Schoen, J.E. Lemons (Eds.) Biomaterials science: an introduction to materials in medicine, Elsevier Amsterdam, 2004.
-
- Zheng YF, Gu XN, Witte F. Biodegradable metals. Mater. Sci. Eng. R. Rep. 2014;77:1–34. doi: 10.1016/j.mser.2014.01.001. - DOI
-
- Fischer J, Profrock D, Hort N, Willumeit R, Feyerabend F. Reprint of: Improved cytotoxicity testing of magnesium materials. Mater. Sci. Eng. B-Adv. 2011;176(20):1773–1777. doi: 10.1016/j.mseb.2011.06.002. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

