Metabolic profiling in children and young adults with autosomal dominant polycystic kidney disease
- PMID: 33758231
- PMCID: PMC7988179
- DOI: 10.1038/s41598-021-84609-8
Metabolic profiling in children and young adults with autosomal dominant polycystic kidney disease
Abstract
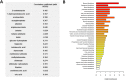
Autosomal dominant polycystic kidney disease (ADPKD) is the most commonly inherited kidney disease. Although children with ADPKD show normal renal function, cyst development is already occurring. In this study, we aimed to identify markers and associated molecular pathways of disease progression in children and young adults with ADPKD. Plasma samples were collected during a 3-year randomized, double-blind, placebo-controlled, phase III clinical trial that was designed to test the efficacy of pravastatin on slowing down ADPKD progression in pediatric patients. Samples from 58 patients were available at baseline and at the 3-year endpoint of the study, respectively. Furthermore, plasma samples from 98 healthy children were used as controls. Metabolomic analysis was performed using liquid chromatography-tandem mass spectrometry and differences in metabolic profiles over time and within study groups were evaluated. While pravastatin therapy led to a decrease in a percent change of total kidney volume (HtTKV) in ADPKD patients, it had minimal effects on metabolite changes. Oxidative stress, endothelial dysfunction, inflammation and immune response were the most affected signaling pathways that distinguished healthy from diseased children. Pathway analysis revealed that metabolites in the arginine metabolism (urea and nitric oxide cycles), asparagine and glutamine metabolism, in the methylation cycle and kynurenine pathway were significantly changed between healthy and children with ADPDK and continued to diverge from the control levels while the disease progressed. Detected metabolite changes were primarily governed by disease progression, and less by pravastatin treatment. Identified metabolic pathways, from arginine and asparagine to kynurenine metabolism could present therapeutic targets and should be further investigated for potential to treat ADPKD progression at an early stage.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Ecder T, Schrier RW. Hypertension in autosomal-dominant polycystic kidney disease: Early occurrence and unique aspects. J. Am. Soc. Nephrol. 2001;12:194–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

