Long-term evolution of the epithelial cell secretome in preclinical 3D models of the human bronchial epithelium
- PMID: 33758289
- PMCID: PMC7988136
- DOI: 10.1038/s41598-021-86037-0
Long-term evolution of the epithelial cell secretome in preclinical 3D models of the human bronchial epithelium
Abstract
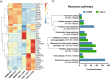
The human bronchial epithelium is the first line of defense against atmospheric particles, pollutants, and respiratory pathogens such as the novel SARS-CoV-2. The epithelial cells form a tight barrier and secrete proteins that are major components of the mucosal immune response. Functional in vitro models of the human lung are essential for screening the epithelial response and assessing the toxicity and barrier crossing of drugs, inhaled particles, and pollutants. However, there is a lack of models to investigate the effect of chronic exposure without resorting to animal testing. Here, we developed a 3D model of the human bronchial epithelium using Calu-3 cell line and demonstrated its viability and functionality for 21 days without subculturing. We investigated the effect of reduced Fetal Bovine Serum supplementation in the basal medium and defined the minimal supplementation needed to maintain a functional epithelium, so that the amount of exogenous serum proteins could be reduced during drug testing. The long-term evolution of the epithelial cell secretome was fully characterized by quantitative mass spectrometry in two preclinical models using Calu-3 or primary NHBE cells. 408 common secreted proteins were identified while significant differences in protein abundance were observed with time, suggesting that 7-10 days are necessary to establish a mature secretome in the Calu-3 model. The associated Reactome pathways highlight the role of the secreted proteins in the immune response of the bronchial epithelium. We suggest this preclinical 3D model can be used to evaluate the long-term toxicity of drugs or particles on the human bronchial epithelium, and subsequently to investigate their effect on the epithelial cell secretions.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Webster MJ, Tarran R. Slippery When Wet: Airway Surface Liquid Homeostasis and Mucus Hydration. Current Topics in Membranes. Elsevier Ltd; 2018. - PubMed
-
- Calderón-Garcidueñas L, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid β-42 and α-synuclein in children and young adult. Toxicol. Pathol. 2008;36:289–310. doi: 10.1177/0192623307313011. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

