Neurodevelopmental outcome of preterm infants enrolled in myo-inositol randomized controlled trial
- PMID: 33758387
- PMCID: PMC8349854
- DOI: 10.1038/s41372-021-01018-5
Neurodevelopmental outcome of preterm infants enrolled in myo-inositol randomized controlled trial
Abstract
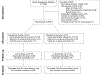
Objective: This study evaluates the 24-month follow-up for the NICHD Neonatal Research Network (NRN) Inositol for Retinopathy Trial.
Study design: Bayley Scales of Infants Development-III and a standardized neurosensory examination were performed in infants enrolled in the main trial. Moderate/severe NDI was defined as BSID-III Cognitive or Motor composite score <85, moderate or severe cerebral palsy, blindness, or hearing loss that prevents communication despite amplification were assessed.
Results: Primary outcome was determined for 605/638 (95%). The mean gestational age was 25.8 ± 1.3 weeks and mean birthweight was 805 ± 192 g. Treatment group did not affect the risk for the composite outcome of death or survival with moderate/severe NDI (60% vs 56%, p = 0.40).
Conclusions: Treatment group did not affect the risk of death or survival with moderate/severe NDI. Despite early termination, this study represents the largest RCT of extremely preterm infants treated with myo-inositol with neurodevelopmental outcome data.
© 2021. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
Conflicts of Interest and Disclosures
None of the authors report any commercial, proprietary, or financial interest in any of the products described in this article. NICHD is the sponsor of the study and holds the investigational new drug (IND) application.
Abbott Nutrition Division, Abbott Laboratories, Columbus, OH, provided the inositol product. They had no role in the: design of the trial; the analyses, interpretation, or writing of the manuscript; or the decision to submit the manuscript for publication. They provided on-site monitoring to assist in quality assurance of the data collection.
Figures
References
-
- Hasan SH, Nishigaki I, Tsutsui Y, Yagi K. Studies on myoinositol. IX. Morphological examinaiton of the effect of massive doses of myoinositol on the liver and kidney of rat. Journal of Nutritional Science and Vitaminology. 1974;20(1):55–58. - PubMed
-
- Hallman M, Bry K, Hoppu K, Lappi M, Pohjavuori M. Inositol Supplementation in Premature Infants with Respiratory Distress Syndrome. New England Journal of Medicine. 1992;326(19):1233–1239. - PubMed
-
- Howlett A, Ohlsson A, Plakkal N. Inositol in preterm infants at risk for or having respiratory distress syndrome. Cochrane Database Syst Rev. 2015(2):CD000366. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UG1 HD068263/HD/NICHD NIH HHS/United States
- UL1 TR93/U.S. Department of Health & Human Services | NIH | National Center for Advancing Translational Sciences (NCATS)
- UL1 TR77/U.S. Department of Health & Human Services | NIH | National Center for Advancing Translational Sciences (NCATS)
- UG1 HD068270/HD/NICHD NIH HHS/United States
- UG1 HD27904/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- UG1 HD68244/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- UL1 TR41/U.S. Department of Health & Human Services | NIH | National Center for Advancing Translational Sciences (NCATS)
- UG1 HD027856/HD/NICHD NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- UG1 HD027904/HD/NICHD NIH HHS/United States
- UG1 HD027880/HD/NICHD NIH HHS/United States
- UG1 HD053109/HD/NICHD NIH HHS/United States
- U10 HD36790/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- UL1 TR003142/TR/NCATS NIH HHS/United States
- UG1 HD27880/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- UG1 HD087226/HD/NICHD NIH HHS/United States
- UG1 HD40689/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- UG1 HD068244/HD/NICHD NIH HHS/United States
- UG1 HD027853/HD/NICHD NIH HHS/United States
- UL1 TR000042/TR/NCATS NIH HHS/United States
- UG1 HD087229/HD/NICHD NIH HHS/United States
- UL1 TR003167/TR/NCATS NIH HHS/United States
- UG1 HD040689/HD/NICHD NIH HHS/United States
- UG1 HD68263/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- UG1 HD27853/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- UG1 HD053089/HD/NICHD NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- UG1 HD53089/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- UL1 TR000442/TR/NCATS NIH HHS/United States
- UG1 HD034216/HD/NICHD NIH HHS/United States
- UG1 HD34216/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- KL2 TR003168/TR/NCATS NIH HHS/United States
- UG1 HD068284/HD/NICHD NIH HHS/United States
- UG1 HD021385/HD/NICHD NIH HHS/United States
- UL1 TR000041/TR/NCATS NIH HHS/United States
- UL1 TR1117/U.S. Department of Health & Human Services | NIH | National Center for Advancing Translational Sciences (NCATS)
- UG1 HD40492/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- UL1 TR454/U.S. Department of Health & Human Services | NIH | National Center for Advancing Translational Sciences (NCATS)
- UG1 HD040492/HD/NICHD NIH HHS/United States
- UG1 HD021364/HD/NICHD NIH HHS/United States
- UG1 HD27851/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- UL1 TR001117/TR/NCATS NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- UG1 HD027851/HD/NICHD NIH HHS/United States
- UL! TR1117/U.S. Department of Health & Human Services | NIH | National Center for Advancing Translational Sciences (NCATS)
- UG1 HD21385/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- U10 HD036790/HD/NICHD NIH HHS/United States
- UG1 HD68284/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
- UL1 TR42/U.S. Department of Health & Human Services | NIH | National Center for Advancing Translational Sciences (NCATS)
- UG1 HD068278/HD/NICHD NIH HHS/United States
- U24 HD095254/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical