The cellular and functional complexity of thermogenic fat
- PMID: 33758402
- PMCID: PMC8159882
- DOI: 10.1038/s41580-021-00350-0
The cellular and functional complexity of thermogenic fat
Abstract
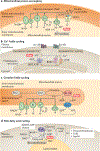
Brown and beige adipocytes are mitochondria-enriched cells capable of dissipating energy in the form of heat. These thermogenic fat cells were originally considered to function solely in heat generation through the action of the mitochondrial protein uncoupling protein 1 (UCP1). In recent years, significant advances have been made in our understanding of the ontogeny, bioenergetics and physiological functions of thermogenic fat. Distinct subtypes of thermogenic adipocytes have been identified with unique developmental origins, which have been increasingly dissected in cellular and molecular detail. Moreover, several UCP1-independent thermogenic mechanisms have been described, expanding the role of these cells in energy homeostasis. Recent studies have also delineated roles for these cells beyond the regulation of thermogenesis, including as dynamic secretory cells and as a metabolic sink. This Review presents our current understanding of thermogenic adipocytes with an emphasis on their development, biological functions and roles in systemic physiology.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



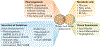

References
-
- Cinti S Obesity, Type 2 Diabetes and the Adipose Organ: A Pictorial Atlas from Research to Clinical Applications 1st edn (Springer, 2017).
-
- Harms M & Seale P Brown and beige fat: development, function and therapeutic potential. Nat. Med 19, 1252–1263 (2013). - PubMed
-
- Lidell ME et al. Evidence for two types of brown adipose tissue in humans. Nat. Med 19, 631–634 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

