Autophagy in tumour immunity and therapy
- PMID: 33758415
- PMCID: PMC8087647
- DOI: 10.1038/s41568-021-00344-2
Autophagy in tumour immunity and therapy
Abstract
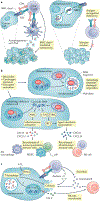
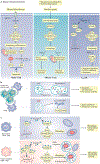
Autophagy is a regulated mechanism that removes unnecessary or dysfunctional cellular components and recycles metabolic substrates. In response to stress signals in the tumour microenvironment, the autophagy pathway is altered in tumour cells and immune cells - thereby differentially affecting tumour progression, immunity and therapy. In this Review, we summarize our current understanding of the immunologically associated roles and modes of action of the autophagy pathway in cancer progression and therapy, and discuss potential approaches targeting autophagy to enhance antitumour immunity and improve the efficacy of current cancer therapy.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures


References
-
- Morishita H & Mizushima N Diverse cellular roles of autophagy. Annu. Rev. Cell Dev. Biol 35, 453–475 (2019). - PubMed
-
- Mizushima N, Yoshimori T & Ohsumi Y The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol 27, 107–132 (2011). - PubMed
-
This detailed review discusses the protein and membrane interactions required for autophagosome formation.
-
- Dikic I & Elazar Z Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol 19, 349–364 (2018). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

