Pharmacokinetics, Bioequivalence and Safety Evaluation of Two Ticagrelor Tablets Under Fasting and Fed Conditions in Healthy Chinese Subjects
- PMID: 33758499
- PMCID: PMC7979338
- DOI: 10.2147/DDDT.S297918
Pharmacokinetics, Bioequivalence and Safety Evaluation of Two Ticagrelor Tablets Under Fasting and Fed Conditions in Healthy Chinese Subjects
Abstract
Purpose: To evaluate the pharmacokinetics (PK), bioequivalence and safety profiles of test drug and reference drug of 90 mg ticagrelor tablets and their main active metabolite AR-C124910XX under fasting and fed conditions.
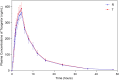
Methods: This was a randomized, open-label, single-dose, two-period, two-sequence, and two-treatment crossover study. Subjects were randomized and evenly administered with a single dose of test drug or reference drug of 90 mg ticagrelor tablets orally under fasting or fed conditions with a 7-day washout period. The primary PK parameters were calculated with non-compartmental model, including peak concentration (Cmax), area under the curve (AUC) from zero to last quantifiable concentration (AUC0-t), and AUC from zero to infinity (AUC0-∞). Bioequivalence was judged by whether the 90% confidence intervals (CIs) of the geometric mean ratio (GMR) of the test/reference drugs were within the predefined range of 80-125%. Adverse events (AEs) were assessed as safety endpoints.
Results: Eighty healthy Chinese subjects (fasting condition: n=40; fed condition: n=40) were enrolled, but two withdrew for personal reasons. As for PK parameters, there was no statistical difference (P>0.05) between the test and reference drugs under both conditions. As for bioequivalence, the 90% CIs of GMR for Cmax, AUC0-t and AUC0-∞ all fell within 80%-125% regardless of food intake or not. No severe adverse events were observed in the study. Chinese clinical trial registration number is ChiCTR1800015091 (http://www.chictr.org.cn).
Conclusion: Our results demonstrated that the test drug and the reference drug of ticagrelor tablets were bioequivalent. The PK and safety profiles were also similar regardless of food intake or not in healthy Chinese subjects.
Keywords: bioequivalence; pharmacokinetics; safety; ticagrelor.
© 2021 Wang et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
-
- Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

