Insights into the role of ERp57 in cancer
- PMID: 33758622
- PMCID: PMC7974888
- DOI: 10.7150/jca.48707
Insights into the role of ERp57 in cancer
Abstract
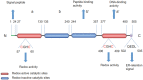
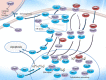
Endoplasmic reticulum resident protein 57 (ERp57) has a molecular weight of 57 kDa, belongs to the protein disulfide-isomerase (PDI) family, and is primarily located in the endoplasmic reticulum (ER). ERp57 functions in the quality control of nascent synthesized glycoproteins, participates in major histocompatibility complex (MHC) class I molecule assembly, regulates immune responses, maintains immunogenic cell death (ICD), regulates the unfolded protein response (UPR), functions as a 1,25-dihydroxy vitamin D3 (1,25(OH)2D3) receptor, regulates the NF-κB and STAT3 pathways, and participates in DNA repair processes and cytoskeletal remodeling. Recent studies have reported ERp57 overexpression in various human cancers, and altered expression and aberrant functionality of ERp57 are associated with cancer growth and progression and changes in the chemosensitivity of cancers. ERp57 may become a potential biomarker and therapeutic target to combat cancer development and chemoresistance. Here, we summarize the available knowledge of the role of ERp57 in cancer and the underlying mechanisms.
Keywords: DNA repair.; ERp57/PDIA3; cancer; immune response; immunogenic cell death; unfolded protein response.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018;68:394–424. - PubMed
-
- Chen Y, Lin MC, Wang H, Chan CY, Jiang L, Ngai SM. et al. Proteomic analysis of EZH2 downstream target proteins in hepatocellular carcinoma. Proteomics. 2007;7:3097–104. - PubMed
-
- Li S, Zhao X, Chang S, Li Y, Guo M, Guan Y. ERp57small interfering RNA silencing can enhance the sensitivity of drugresistant human ovarian cancer cells to paclitaxel. International journal of oncology. 2019;54:249–60. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

