This is a preprint.
Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva
- PMID: 33758842
- PMCID: PMC7987001
- DOI: 10.1101/2021.03.11.434841
Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva
Update in
-
Antibody Responses to SARS-CoV-2 mRNA Vaccines Are Detectable in Saliva.Pathog Immun. 2021 Jun 7;6(1):116-134. doi: 10.20411/pai.v6i1.441. eCollection 2021. Pathog Immun. 2021. PMID: 34136730 Free PMC article.
Abstract
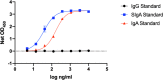
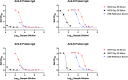
Vaccines are critical for curtailing the COVID-19 pandemic (1, 2). In the USA, two highly protective mRNA vaccines are available: BNT162b2 from Pfizer/BioNTech and mRNA-1273 from Moderna (3, 4). These vaccines induce antibodies to the SARS-CoV-2 S-protein, including neutralizing antibodies (NAbs) predominantly directed against the Receptor Binding Domain (RBD) (1-4). Serum NAbs are induced at modest levels within ~1 week of the first dose, but their titers are strongly boosted by a second dose at 3 (BNT162b2) or 4 weeks (mRNA-1273) (3, 4). SARS-CoV-2 is most commonly transmitted nasally or orally and infects cells in the mucosae of the respiratory and to some extent also the gastrointestinal tract (5). Although serum NAbs may be a correlate of protection against COVID-19, mucosal antibodies might directly prevent or limit virus acquisition by the nasal, oral and conjunctival routes (5). Whether the mRNA vaccines induce mucosal immunity has not been studied. Here, we report that antibodies to the S-protein and its RBD are present in saliva samples from mRNA-vaccinated healthcare workers (HCW). Within 1-2 weeks after their second dose, 37/37 and 8/8 recipients of the Pfizer and Moderna vaccines, respectively, had S-protein IgG antibodies in their saliva, while IgA was detected in a substantial proportion. These observations may be relevant to vaccine-mediated protection from SARS-CoV-2 infection and disease.
Keywords: COVID-19; RBD; S-protein; SARS-CoV-2; saliva.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures





References
-
- Krammer F. SARS-CoV-2 vaccines in development. Nature 586, 516–527 (2020). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
