This is a preprint.
Memory B cell repertoire for recognition of evolving SARS-CoV-2 spike
- PMID: 33758863
- PMCID: PMC7987022
- DOI: 10.1101/2021.03.10.434840
Memory B cell repertoire for recognition of evolving SARS-CoV-2 spike
Update in
-
Memory B cell repertoire for recognition of evolving SARS-CoV-2 spike.Cell. 2021 Sep 16;184(19):4969-4980.e15. doi: 10.1016/j.cell.2021.07.025. Epub 2021 Jul 23. Cell. 2021. PMID: 34332650 Free PMC article.
Abstract
Memory B cell reserves can generate protective antibodies against repeated SARS-CoV-2 infections, but with an unknown reach from original infection to antigenically drifted variants. We charted memory B cell receptor-encoded monoclonal antibodies (mAbs) from 19 COVID-19 convalescent subjects against SARS-CoV-2 spike (S) and found 7 major mAb competition groups against epitopes recurrently targeted across individuals. Inclusion of published and newly determined structures of mAb-S complexes identified corresponding epitopic regions. Group assignment correlated with cross-CoV-reactivity breadth, neutralization potency, and convergent antibody signatures. mAbs that competed for binding the original S isolate bound differentially to S variants, suggesting the protective importance of otherwise-redundant recognition. The results furnish a global atlas of the S-specific memory B cell repertoire and illustrate properties conferring robustness against emerging SARS-CoV-2 variants.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures


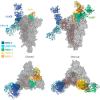

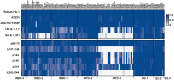
References
-
- Victora G. D., Nussenzweig M. C., Germinal centers. Annu Rev Immunol 30, 429–457 (2012). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
