This is a preprint.
Serological surveys to estimate cumulative incidence of SARS-CoV-2 infection in adults (Sero-MAss study), Massachusetts, July-August 2020: a mail-based cross-sectional study
- PMID: 33758898
- PMCID: PMC7987057
- DOI: 10.1101/2021.03.05.21249174
Serological surveys to estimate cumulative incidence of SARS-CoV-2 infection in adults (Sero-MAss study), Massachusetts, July-August 2020: a mail-based cross-sectional study
Update in
-
Serological surveys to estimate cumulative incidence of SARS-CoV-2 infection in adults (Sero-MAss study), Massachusetts, July-August 2020: a mail-based cross-sectional study.BMJ Open. 2021 Aug 17;11(8):e051157. doi: 10.1136/bmjopen-2021-051157. BMJ Open. 2021. PMID: 34404716 Free PMC article.
Abstract
Background: The SARS-CoV-2 pandemic is an unprecedented global health crisis. The state of Massachusetts was especially impacted during the initial stages; however, the extent of asymptomatic transmission remains poorly understood due to limited asymptomatic testing in the "first wave." To address this gap, a geographically representative and contact-free seroprevalence survey was conducted in July-August 2020, to estimate prior undetected SARS-CoV-2 infections.
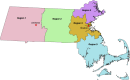
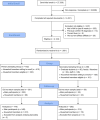
Methods: Students, faculty, librarians and staff members at the University of Massachusetts, Amherst without a previous COVID-19 diagnosis were invited to participate in this study along with one member of their household in June 2020. Two separate sampling frames were generated from administrative lists: all undergraduates and their household members (primary sampling group) were randomly selected with probability proportional to population size. All staff, faculty, graduate students and librarians (secondary sampling group) were selected as a simple random sample. After informed consent and a socio-behavioral survey, participants were mailed test kits and asked to return self-collected dried blood spot (DBS) samples. Samples were analyzed via ELISA for anti-SARS-CoV-2 IgG antibodies, and then IgM antibodies if IgG-positive. Seroprevalence estimates were adjusted for survey non-response. Binomial models were used to assess factors associated with seropositivity in both sample groups separately.
Results: Approximately 27,000 persons were invited via email to assess eligibility. Of the 1,001 individuals invited to participate in the study, 762 (76%) returned blood samples for analysis. In the primary sampling group 548 returned samples, of which 230 enrolled a household member. Within the secondary sampling group of 214 individuals, 79 enrolled a household member. In the primary sample group, 36 (4.6%) had IgG antibodies detected for an estimated weighed prevalence for this population of 5.3% (95% CI: 3.5 to 8.0). In the secondary sampling group, 10 (3.4%) of 292 individuals had IgG antibodies detected for an estimated adjusted prevalence of 4.0% (95% CI: 2.2 to 7.4). No samples were IgM positive. No association was found in either sample group between seropositivity and self-reported work duties or customer-facing hours. In the primary sampling group, self-reported febrile illness since Feb 2020, male sex, and minority race (Black or American Indian/Alaskan Native) were associated with seropositivity. No factors except geographic regions within the state were associated with evidence of prior SARS-CoV-2 infection in the secondary sampling group.
Interpretation: This study provides insight into the seroprevalence of university-related populations and their household members across the state of Massachusetts during the summer of 2020 of the pandemic and helps to fill a critical gap in estimating the levels of sub-clinical and asymptomatic infection. Estimates like these can be used to calibrate models that estimate levels of population immunity over time to inform public health interventions and policy.
Conflict of interest statement
COI statements None of the authors report any conflicts of interest, financial or non-financial, regarding this study.
Figures
Similar articles
-
Serological surveys to estimate cumulative incidence of SARS-CoV-2 infection in adults (Sero-MAss study), Massachusetts, July-August 2020: a mail-based cross-sectional study.BMJ Open. 2021 Aug 17;11(8):e051157. doi: 10.1136/bmjopen-2021-051157. BMJ Open. 2021. PMID: 34404716 Free PMC article.
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
Seroprevalence of SARS-CoV-2-specific anti-spike IgM, IgG, and anti-nucleocapsid IgG antibodies during the second wave of the pandemic: A population-based cross-sectional survey across Kashmir, India.Front Public Health. 2022 Oct 6;10:967447. doi: 10.3389/fpubh.2022.967447. eCollection 2022. Front Public Health. 2022. PMID: 36276377 Free PMC article.
-
Seroprevalence of anti-SARS-CoV-2 IgG Antibodies in the Staff of a Public School System in the Midwestern United States.medRxiv [Preprint]. 2020 Oct 27:2020.10.23.20218651. doi: 10.1101/2020.10.23.20218651. medRxiv. 2020. Update in: PLoS One. 2021 Jun 10;16(6):e0243676. doi: 10.1371/journal.pone.0243676. PMID: 33140066 Free PMC article. Updated. Preprint.
-
Strengthening serological studies: the need for greater geographical diversity, biobanking, and data-accessibility.Trends Microbiol. 2025 Jan 15:S0966-842X(24)00322-6. doi: 10.1016/j.tim.2024.12.006. Online ahead of print. Trends Microbiol. 2025. PMID: 39818508 Review.
References
-
- Massachusetts Department of Public Health COVID-19 Dashboard. Department of Public Health, Massachusetts; 2020. Accessed October 25, 2020. https://www.mass.gov/doc/covid-19-dashboard-july-1-2020/download
-
- Gronvall G, Connell N, Kobokovich A, et al. Developing a National Strategy for Serology (Antibody Testing) in the United States. The Johns Hopkins Center for Health Security; 2020. https://www.centerforhealthsecurity.org/our-work/pubs_archive/pubs-pdfs/...
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous