Letrozole-Induced Polycystic Ovary Syndrome Attenuates Cystathionine-β Synthase mRNA and Protein Abundance in the Ovaries of Female Sprague Dawley Rats
- PMID: 33758914
- PMCID: PMC8169814
- DOI: 10.1093/jn/nxab038
Letrozole-Induced Polycystic Ovary Syndrome Attenuates Cystathionine-β Synthase mRNA and Protein Abundance in the Ovaries of Female Sprague Dawley Rats
Abstract
Background: Polycystic ovary syndrome (PCOS) is an endocrine disorder that affects 10% of reproductive-aged women and leads to hyperandrogenism, anovulation, and infertility. PCOS has been associated with elevated serum homocysteine as well as altered methylation status; however, characterization of one-carbon metabolism (OCM) in PCOS remains incomplete.
Objectives: The aim of our research was to assess OCM in a letrozole-induced Sprague Dawley rat model of PCOS.
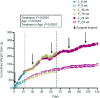
Methods: Five-week-old female rats (n = 36) were randomly assigned to letrozole [0.9 mg/kg body weight (BW)] treatment or vehicle (carboxymethylcellulose) control that was administered via subcutaneously implanted slow-release pellets every 30 d. For both treatment groups, 12 rats were randomly assigned to be euthanized during proestrus at one of the following time points: 8, 16, or 24 wk of age. Daily BW was measured and estrous cyclicity was monitored during the last 30 d of the experimental period. Ovaries were collected to assess mRNA and protein abundance of OCM enzymes.
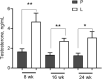
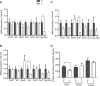
Results: Letrozole-induced rats exhibited 1.9-fold higher cumulative BW gain compared with control rats across all age groups (P < 0.0001). Letrozole reduced the time spent at proestrus (P = 0.0001) and increased time in metestrus (P < 0.0001) of the estrous cycle. Cystathionine β-synthase (Cbs) mRNA abundance was reduced in the letrozole-induced rats at 16 (59%; P < 0.05) and 24 (77%; P < 0.01) wk of age. In addition, CBS protein abundance was 32% lower in 8-wk-old letrozole-induced rats (P = 0.02). Interestingly, betaine-homocysteine S-methyltransferase mRNA abundance increased as a function of age in letrozole-induced rats (P = 0.03).
Conclusion: These data demonstrate that letrozole-induced PCOS Sprague Dawley rats temporally decrease the ovarian abundance of Cbs mRNA and protein in the early stages of PCOS.
Keywords: cystathionine-β synthase; letrozole; one-carbon metabolism; polycystic ovary syndrome; rat.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures


References
-
- Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, Dabadghao P, Darendeliler F, Elbarbary NS, Gambineri Aet al. . An international consortium update: pathophysiology, diagnosis, and treatment of polycystic ovarian syndrome in adolescence. Hormone Res Paediatr. 2017;88:371–95. - PubMed
-
- Wu LLY, Norman RJ, Robker RL. The impact of obesity on oocytes: evidence for lipotoxicity mechanisms. Reprod Fertil Dev. 2011;24:29–34. - PubMed
-
- Sander VA, Hapon MB, Sícaro L, Lombardi EP, Jahn GA, Motta AB. Alterations of folliculogenesis in women with polycystic ovary syndrome. J Steroid Biochem Mol Biol. 2011;124:58–64. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

