Chemoreceptor mechanisms regulating CO2 -induced arousal from sleep
- PMID: 33759184
- PMCID: PMC8830133
- DOI: 10.1113/JP281305
Chemoreceptor mechanisms regulating CO2 -induced arousal from sleep
Abstract
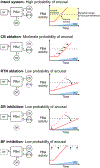
Arousal from sleep in response to CO2 is a life-preserving reflex that enhances ventilatory drive and facilitates behavioural adaptations to restore eupnoeic breathing. Recurrent activation of the CO2 -arousal reflex is associated with sleep disruption in obstructive sleep apnoea. In this review we examine the role of chemoreceptors in the carotid bodies, the retrotrapezoid nucleus and serotonergic neurons in the dorsal raphe in the CO2 -arousal reflex. We also provide an overview of the supra-medullary structures that mediate CO2 -induced arousal. We propose a framework for the CO2 -arousal reflex in which the activity of the chemoreceptors converges in the parabrachial nucleus to trigger cortical arousal.
Keywords: arousal threshold; interoception; obstructive sleep apnea; optogenetic; respiration; sleep-wake behavior.
© 2021 The Authors. The Journal of Physiology © 2021 The Physiological Society.
Conflict of interest statement
Competing interests
The authors declare they have no competing interests.
Figures

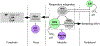
References
-
- Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C & Lyonnet S (2003). Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 33, 459–461. - PubMed
-
- Banzett RB, Lansing RW, Evans KC & Shea SA (1996). Stimulus-response characteristics of CO2-induced air hunger in normal subjects. Respir Physiol 103, 19–31. - PubMed
-
- Berquin P, Bodineau L, Gros F & Larnicol N (2000). Brainstem and hypothalamic areas involved in respiratory chemoreflexes: a Fos study in adult rats. Brain Res 857, 30–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

