Selection of Antiobesity Medications Based on Phenotypes Enhances Weight Loss: A Pragmatic Trial in an Obesity Clinic
- PMID: 33759389
- PMCID: PMC8168710
- DOI: 10.1002/oby.23120
Selection of Antiobesity Medications Based on Phenotypes Enhances Weight Loss: A Pragmatic Trial in an Obesity Clinic
Erratum in
-
Selection of antiobesity medications based on phenotypes enhances weight loss: A pragmatic trial in an obesity clinic.Obesity (Silver Spring). 2021 Sep;29(9):1565-1566. doi: 10.1002/oby.23236. Epub 2021 Jun 22. Obesity (Silver Spring). 2021. PMID: 34156162 Free PMC article. No abstract available.
-
Selection of antiobesity medications based on phenotypes enhances weight loss: a pragmatic trial in an obesity clinic.Obesity (Silver Spring). 2022 Jul;30(7):1521. doi: 10.1002/oby.23498. Epub 2022 May 25. Obesity (Silver Spring). 2022. PMID: 35611684 No abstract available.
Abstract
Objective: Little is known about the predictors of response to obesity interventions.
Methods: In 450 participants with obesity, body composition, resting energy expenditure, satiety, satiation, eating behavior, affect, and physical activity were measured by validated studies and questionnaires. These variables were used to classify obesity phenotypes. Subsequently, in a 12-month, pragmatic, real-world trial performed in a weight management center, 312 patients were randomly assigned to phenotype-guided treatment or non-phenotype-guided treatment with antiobesity medications: phentermine, phentermine/topiramate, bupropion/naltrexone, lorcaserin, and liraglutide. The primary outcome was weight loss at 12 months.
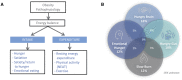
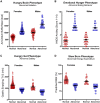
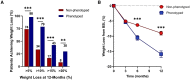
Results: Four phenotypes of obesity were identified in 383 of 450 participants (85%): hungry brain (abnormal satiation), emotional hunger (hedonic eating), hungry gut (abnormal satiety), and slow burn (decreased metabolic rate). In 15% of participants, no phenotype was identified. Two or more phenotypes were identified in 27% of patients. In the pragmatic clinical trial, the phenotype-guided approach was associated with 1.75-fold greater weight loss after 12 months with mean weight loss of 15.9% compared with 9.0% in the non-phenotype-guided group (difference -6.9% [95% CI -9.4% to -4.5%], P < 0.001), and the proportion of patients who lost >10% at 12 months was 79% in the phenotype-guided group compared with 34% with non-phenotype-guided treatment group.
Conclusions: Biological and behavioral phenotypes elucidate human obesity heterogeneity and can be targeted pharmacologically to enhance weight loss.
Trial registration: ClinicalTrials.gov NCT03374956.
© 2021 The Obesity Society.
Conflict of interest statement
Phenomix Sciences has obtained an exclusive license to AA and MC’s biomarker technology, submitted patent, and know‐how to develop a biomarker to predict response to obesity pharmacotherapy. Additionally, AA is a stockholder in Gila Therapeutics and Phenomix Sciences; he serves as a consultant for Rhythm Pharmaceuticals and General Mills. MC is a stockholder in Phenomix Sciences and Enterin and serves as a consultant to Takeda, Allergan, Kallyope, GlaxoSmithKline, Rhythm, and Arena with compensation to Mayo Clinic. MMC is a consultant for Roche Diabetes Care GmbH. BAD has served as a consultant for Boston Scientific, Metamodix, BFKW, DyaMx, and USGI Medical; has received research support for Boston Scientific, Apollo Endosurgery, USGI, Spatz Medical, GI Dynamics, Caim Diagnostics, Aspire Bariatrics, and Medtronic; and has been a speaker for Johnson & Johnson, Endogastric Solutions, and Olympus. The other authors declared no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

