Dual Antibody-Conjugated Amyloid Nanorods to Promote Selective Cell-Cell Interactions
- PMID: 33759489
- PMCID: PMC9262253
- DOI: 10.1021/acsami.0c21996
Dual Antibody-Conjugated Amyloid Nanorods to Promote Selective Cell-Cell Interactions
Abstract
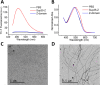
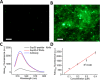
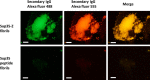
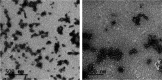
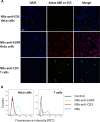
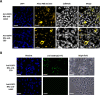
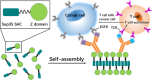
Grafting biomolecules on nanostructures' surfaces is an increasingly used strategy to target pathogenic cells, with both diagnostic and therapeutic applications. However, nanomaterials monofunctionalized by conjugating a single type of ligand find limited uses in pathologies/therapies that require two or more targets/receptors to be targeted and/or activated with a single molecular entity simultaneously. Therefore, multivalent nanomaterials for dual- or multitargeting are attracting significant interest. This study provides a proof of concept of such nanostructures. We have recently developed a modular methodology that allows obtaining amyloid-based materials decorated with active globular domains. Here, this approach is exploited to generate functional amyloid fibrils displaying antibody capture moieties. A high antibody binding affinity and capacity for the resulting nanofibrils, whose size can be manipulated to obtain homogeneous nanorods with high biocompatibility, are demonstrated. These nanorods are then used for specific antibody-mediated targeting of different cell types. Simultaneous conjugation of these nanorods with different antibodies allows obtaining a mimic of a bispecific antibody that redirects T lymphocytes to tumoral cells, holding high potential for immunotherapy. Overall, the work illustrates a modular and straightforward strategy to obtain preparative quantities of multivalent antibody-functionalized nanomaterials with multitargeting properties without the need for covalent modification.
Keywords: amyloid; antibody; dual- or multitargeting; multivalency; nanomaterials; nanorods.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Bispecific Antibodies and Antibody-Drug Conjugates for Cancer Therapy: Technological Considerations.Biomolecules. 2020 Feb 26;10(3):360. doi: 10.3390/biom10030360. Biomolecules. 2020. PMID: 32111076 Free PMC article. Review.
-
FHR4-based immunoconjugates direct complement-dependent cytotoxicity and phagocytosis towards HER2-positive cancer cells.Mol Oncol. 2019 Dec;13(12):2531-2553. doi: 10.1002/1878-0261.12554. Epub 2019 Sep 30. Mol Oncol. 2019. PMID: 31365168 Free PMC article.
-
Pharmacokinetic/pharmacodynamic relationship of therapeutic monoclonal antibodies used in oncology: Part 1, monoclonal antibodies, antibody-drug conjugates and bispecific T-cell engagers.Eur J Cancer. 2020 Mar;128:107-118. doi: 10.1016/j.ejca.2020.01.005. Epub 2020 Feb 6. Eur J Cancer. 2020. PMID: 32037061 Review.
-
Synthesis of precision antibody conjugates using proximity-induced chemistry.Theranostics. 2021 Aug 27;11(18):9107-9117. doi: 10.7150/thno.62444. eCollection 2021. Theranostics. 2021. PMID: 34522229 Free PMC article.
-
Multifunctional antibody-conjugated coiled-coil protein nanoparticles for selective cell targeting.Acta Biomater. 2021 Sep 1;131:472-482. doi: 10.1016/j.actbio.2021.06.040. Epub 2021 Jun 27. Acta Biomater. 2021. PMID: 34192568
Cited by
-
gp120-derived amyloidogenic peptides form amyloid fibrils that increase HIV-1 infectivity.Cell Mol Immunol. 2024 May;21(5):479-494. doi: 10.1038/s41423-024-01144-y. Epub 2024 Mar 5. Cell Mol Immunol. 2024. PMID: 38443447 Free PMC article.
-
OligoBinders: Bioengineered Soluble Amyloid-like Nanoparticles to Bind and Neutralize SARS-CoV-2.ACS Appl Mater Interfaces. 2023 Mar 8;15(9):11444-11457. doi: 10.1021/acsami.2c18305. Epub 2023 Feb 22. ACS Appl Mater Interfaces. 2023. PMID: 36890692 Free PMC article.
-
Novel, Inexpensive, and Scalable Amyloid Fibril Formation Method.Materials (Basel). 2022 Feb 26;15(5):1766. doi: 10.3390/ma15051766. Materials (Basel). 2022. PMID: 35268997 Free PMC article.
-
Local environment in biomolecular condensates modulates enzymatic activity across length scales.Nat Commun. 2024 Apr 18;15(1):3322. doi: 10.1038/s41467-024-47435-w. Nat Commun. 2024. PMID: 38637545 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

