PepT1-knockout mice harbor a protective metabolome beneficial for intestinal wound healing
- PMID: 33759563
- PMCID: PMC8202197
- DOI: 10.1152/ajpgi.00299.2020
PepT1-knockout mice harbor a protective metabolome beneficial for intestinal wound healing
Abstract
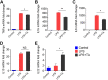
Genetic knockout (KO) of peptide transporter-1 (PepT1) protein is known to provide resistance to acute colitis and colitis-associated cancer (CAC) in mouse models. However, it was unclear which molecule(s) or pathway(s) formed the basis for these protective effects. Recently, we demonstrated that the PepT1-/- microbiota is sufficient to protect against colitis and CAC. Given that PepT1 KO alters the gut microbiome and thereby changes the intestinal metabolites that are ultimately reflected in the feces, we investigated the fecal metabolites of our PepT1 KO mice. Using a liquid chromatography-mass spectrometry (LC-MS)-based untargeted-metabolomics technique, we found that the fecal metabolites were significantly different between the KO and normal wild-type (WT) mice. Among the altered fecal metabolites, tuberonic acid (TA) was sevenfold higher in KO mouse feces than in WT mouse feces. Accordingly, we studied whether the increased TA could direct an anti-inflammatory effect. Using in vitro models, we discovered that TA not only prevented lipopolysaccharide (LPS)-induced inflammation in macrophages but also improved the epithelial cell healing processes. Our results suggest that TA, and possibly other fecal metabolites, play a crucial role in the pathway(s) associated with the anticolitis effects of PepT1 KO.NEW & NOTEWORTHY Fecal metabolites were significantly different between the KO and normal wild-type (WT) mice. One fecal metabolite, tuberonic acid (TA), was sevenfold higher in KO mouse feces than in WT mouse feces. TA prevented lipopolysaccharide (LPS)-induced inflammation in macrophages and improved the epithelial cell healing process.
Keywords: fecal metabolites; inflammatory bowel disease; knockout; peptide transporter 1 (PepT1); tuberonic acid.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
-
- Wuensch T, Schulz S, Ullrich S, Lill N, Stelzl T, Rubio-Aliaga I, Loh G, Chamaillard M, Haller D, Daniel H. The peptide transporter PEPT1 is expressed in distal colon in rodents and humans and contributes to water absorption. Am J Physiol Gastrointest Liver Physiol 305: G66–G73, 2013. doi: 10.1152/ajpgi.00491.2012. - DOI - PubMed
-
- Zucchelli M, Torkvist L, Bresso F, Halfvarson J, Hellquist A, Anedda F, Assadi G, Lindgren GB, Svanfeldt M, Janson M, Noble CL, Pettersson S, Lappalainen M, Paavola-Sakki P, Halme L, Farkkila M, Turunen U, Satsangi J, Kontula K, Lofberg R, Kere J, D'Amato M. PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis 15: 1562–1569, 2009. doi: 10.1002/ibd.20963. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

