Blockade of PD-1 decreases neutrophilic inflammation and lung damage in experimental COPD
- PMID: 33759577
- PMCID: PMC8424521
- DOI: 10.1152/ajplung.00121.2020
Blockade of PD-1 decreases neutrophilic inflammation and lung damage in experimental COPD
Abstract
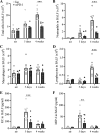
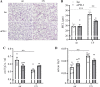
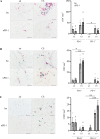
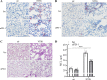
Chronic obstructive lung disease (COPD) and lung cancer are both caused by smoking and often occur as comorbidity. The programmed cell death protein 1/programmed cell death ligand 1 (PD-1/PD-L1) axis is an important canonic immunoregulatory pathway, and antibodies that specifically block PD-1 or PD-L1 have demonstrated efficacy as therapeutic agents for non-small cell lung cancer. The role of the PD-1/PD-L1 axis in the pathogenesis of COPD is unknown. Here, we analyzed the function of the PD-1/PD-L1 axis in preclinical COPD models and evaluated the concentrations of PD-1 and PD-L1 in human serum and bronchoalveolar lavage (BAL) fluids as biomarkers for COPD. Anti-PD-1 treatment decreased lung damage and neutrophilic inflammation in mice chronically exposed to cigarette smoke (CS) or nontypeable Haemophilus influenzae (NTHi). Ex vivo stimulated macrophages obtained from anti-PD-1-treated mice released reduced amounts of inflammatory cytokines. PD-L1 concentrations correlated positively with PD-1 concentrations in human serum and BAL fluids. Lung sections obtained from patients with COPD stained positive for PD-L1. Our data indicate that the PD-1/PD-L1 axis is involved in developing inflammation and tissue destruction in COPD. Inflammation-induced activation of the PD-1 pathway may contribute to disease progression.
Keywords: COPD; PD-1; PD-L1; inflammation; lung damage; macrophages.
Conflict of interest statement
No conflicts of interest, financial or otherwise are declared by the authors.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

