Glia actively sculpt sensory neurons by controlled phagocytosis to tune animal behavior
- PMID: 33759761
- PMCID: PMC8079151
- DOI: 10.7554/eLife.63532
Glia actively sculpt sensory neurons by controlled phagocytosis to tune animal behavior
Abstract
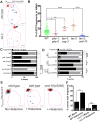
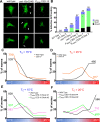
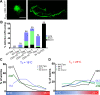
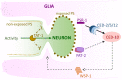
Glia in the central nervous system engulf neuron fragments to remodel synapses and recycle photoreceptor outer segments. Whether glia passively clear shed neuronal debris or actively prune neuron fragments is unknown. How pruning of single-neuron endings impacts animal behavior is also unclear. Here, we report our discovery of glia-directed neuron pruning in Caenorhabditis elegans. Adult C. elegans AMsh glia engulf sensory endings of the AFD thermosensory neuron by repurposing components of the conserved apoptotic corpse phagocytosis machinery. The phosphatidylserine (PS) flippase TAT-1/ATP8A functions with glial PS-receptor PSR-1/PSR and PAT-2/α-integrin to initiate engulfment. This activates glial CED-10/Rac1 GTPase through the ternary GEF complex of CED-2/CrkII, CED-5/DOCK180, CED-12/ELMO. Execution of phagocytosis uses the actin-remodeler WSP-1/nWASp. This process dynamically tracks AFD activity and is regulated by temperature, the AFD sensory input. Importantly, glial CED-10 levels regulate engulfment rates downstream of neuron activity, and engulfment-defective mutants exhibit altered AFD-ending shape and thermosensory behavior. Our findings reveal a molecular pathway underlying glia-dependent engulfment in a peripheral sense-organ and demonstrate that glia actively engulf neuron fragments, with profound consequences on neuron shape and animal sensory behavior.
Keywords: C. elegans; Glia; Rac1 small GTPase; cell biology; neuroscience; phagocytosis; pruning; sensory systems; thermotaxis.
Plain language summary
Neurons are tree-shaped cells that receive information through endings connected to neighbouring cells or the environment. Controlling the size, number and location of these endings is necessary to ensure that circuits of neurons get precisely the right amount of input from their surroundings. Glial cells form a large portion of the nervous system, and they are tasked with supporting, cleaning and protecting neurons. In humans, part of their duties is to ‘eat’ (or prune) unnecessary neuron endings. In fact, this role is so important that defects in glial pruning are associated with conditions such as Alzheimer’s disease. Yet it is still unknown how pruning takes place, and in particular whether it is the neuron or the glial cell that initiates the process. To investigate this question, Raiders et al. enlisted the common laboratory animal Caenorhabditis elegans, a tiny worm with a simple nervous system where each neuron has been meticulously mapped out. First, the experiments showed that glial cells in C. elegans actually prune the endings of sensory neurons. Focusing on a single glia-neuron pair then revealed that the glial cell could trim the endings of a living neuron by redeploying the same molecular machinery it uses to clear dead cell debris. Compared to this debris-clearing activity, however, the glial cell takes a more nuanced approach to pruning: specifically, it can adjust the amount of trimming based on the activity load of the neuron. When Raiders et al. disrupted the glial pruning for a single temperature-sensing neuron, the worm lost its normal temperature preferences; this demonstrated how the pruning activity of a single glial cell can be linked to behavior. Taken together the experiments showcase how C. elegans can be used to study glial pruning. Further work using this model could help to understand how disease emerges when glial cells cannot perform their role, and to spot the genetic factors that put certain individuals at increased risk for neurological and sensory disorders.
© 2021, Raiders et al.
Conflict of interest statement
SR, EB, AB, SM, MK, SS, AS No competing interests declared
Figures


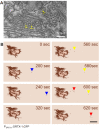
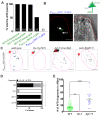
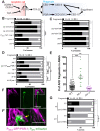

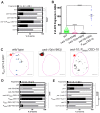

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

