Electron microscopy shows that binding of monoclonal antibody PT25-2 primes integrin αIIbβ3 for ligand binding
- PMID: 33760023
- PMCID: PMC8045492
- DOI: 10.1182/bloodadvances.2020004166
Electron microscopy shows that binding of monoclonal antibody PT25-2 primes integrin αIIbβ3 for ligand binding
Abstract
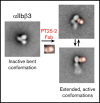
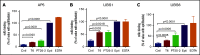
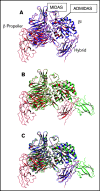
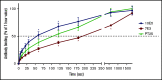
The murine monoclonal antibody (mAb) PT25-2 induces αIIbβ3 to bind ligand and initiate platelet aggregation. The underlying mechanism is unclear, because previous mutagenesis studies suggested that PT25-2 binds to the αIIb β propeller, a site distant from the Arg-Gly-Asp-binding pocket. To elucidate the mechanism, we studied the αIIbβ3-PT25-2 Fab complex by negative-stain and cryo-electron microscopy (EM). We found that PT25-2 binding results in αIIbβ3 partially exposing multiple ligand-induced binding site epitopes and adopting extended conformations without swing-out of the β3 hybrid domain. The cryo-EM structure showed PT25-2 binding to the αIIb residues identified by mutagenesis but also to 2 additional regions. Overlay of the cryo-EM structure with the bent αIIbβ3 crystal structure showed that binding of PT25-2 creates clashes with the αIIb calf-1/calf-2 domains, suggesting that PT25-2 selectively binds to partially or fully extended receptor conformations and prevents a return to its bent conformation. Kinetic studies of the binding of PT25-2 compared with mAbs 10E5 and 7E3 support this hypothesis. We conclude that PT25-2 induces αIIbβ3 ligand binding by binding to extended conformations and by preventing the interactions between the αIIb and β3 leg domains and subsequently the βI and β3 leg domains required for the bent-closed conformation.
© 2021 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: B.S.C. has royalty interests in abciximab (Centocor) through the Research Foundation of the State University of New York and the VerifyNow Assays (Accumetrics/Instrumentation Laboratories), and is an equity holder and consultant to Scholar Rock, CeleCor Therapeutics, and Pulmoquine Therapeutics. The remaining authors declare no competing financial interests.
Figures



Similar articles
-
An αIIbβ3 monoclonal antibody traps a semiextended conformation and allosterically inhibits large ligand binding.Blood Adv. 2024 Aug 27;8(16):4398-4409. doi: 10.1182/bloodadvances.2024013177. Blood Adv. 2024. PMID: 38968144 Free PMC article.
-
Integrin beta3 regions controlling binding of murine mAb 7E3: implications for the mechanism of integrin alphaIIbbeta3 activation.Proc Natl Acad Sci U S A. 2004 Sep 7;101(36):13114-20. doi: 10.1073/pnas.0404201101. Epub 2004 Jul 26. Proc Natl Acad Sci U S A. 2004. PMID: 15277669 Free PMC article.
-
Effects of limiting extension at the alphaIIb genu on ligand binding to integrin alphaIIbbeta3.J Biol Chem. 2010 Jun 4;285(23):17604-13. doi: 10.1074/jbc.M110.107763. Epub 2010 Apr 2. J Biol Chem. 2010. PMID: 20363746 Free PMC article.
-
Regulation of integrins in platelets.Biopolymers. 2015 Jul;104(4):323-33. doi: 10.1002/bip.22679. Biopolymers. 2015. PMID: 26010651 Review.
-
Beta3 tyrosine phosphorylation in alphaIIbbeta3 (platelet membrane GP IIb-IIIa) outside-in integrin signaling.Thromb Haemost. 2001 Jul;86(1):246-58. Thromb Haemost. 2001. PMID: 11487013 Review.
Cited by
-
Organization, dynamics and mechanoregulation of integrin-mediated cell-ECM adhesions.Nat Rev Mol Cell Biol. 2023 Feb;24(2):142-161. doi: 10.1038/s41580-022-00531-5. Epub 2022 Sep 27. Nat Rev Mol Cell Biol. 2023. PMID: 36168065 Free PMC article. Review.
-
An αIIbβ3 ligand-mimetic murine monoclonal antibody that produces platelet activation by engaging the FcγIIa receptor.Blood Adv. 2025 Jul 22;9(14):3518-3529. doi: 10.1182/bloodadvances.2024015368. Blood Adv. 2025. PMID: 40101235 Free PMC article.
-
An αIIbβ3 monoclonal antibody traps a semiextended conformation and allosterically inhibits large ligand binding.Blood Adv. 2024 Aug 27;8(16):4398-4409. doi: 10.1182/bloodadvances.2024013177. Blood Adv. 2024. PMID: 38968144 Free PMC article.
-
Platelet binding to polymerizing fibrin is avidity driven and requires activated αIIbβ3 but not fibrin cross-linking.Blood Adv. 2021 Oct 26;5(20):3986-4002. doi: 10.1182/bloodadvances.2021005142. Blood Adv. 2021. PMID: 34647980 Free PMC article.
References
-
- Tokuhira M, Handa M, Kamata T, et al. . A novel regulatory epitope defined by a murine monoclonal antibody to the platelet GPIIb-IIIa complex (alpha IIb beta 3 integrin). Thromb Haemost. 1996;76(6):1038-1046. - PubMed
-
- Tadokoro S, Tomiyama Y, Honda S, et al. . A Gln747–>Pro substitution in the IIb subunit is responsible for a moderate IIbbeta3 deficiency in Glanzmann thrombasthenia. Blood. 1998;92(8):2750-2758. - PubMed
-
- Basani RB, French DL, Vilaire G, et al. . A naturally occurring mutation near the amino terminus of alphaIIb defines a new region involved in ligand binding to alphaIIbbeta3. Blood. 2000;95(1):180-188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

