CSPα reduces aggregates and rescues striatal dopamine release in α-synuclein transgenic mice
- PMID: 33760024
- PMCID: PMC8320296
- DOI: 10.1093/brain/awab076
CSPα reduces aggregates and rescues striatal dopamine release in α-synuclein transgenic mice
Abstract
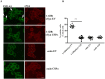
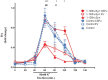
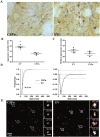
α-Synuclein aggregation at the synapse is an early event in Parkinson's disease and is associated with impaired striatal synaptic function and dopaminergic neuronal death. The cysteine string protein (CSPα) and α-synuclein have partially overlapping roles in maintaining synaptic function and mutations in each cause neurodegenerative diseases. CSPα is a member of the DNAJ/HSP40 family of co-chaperones and like α-synuclein, chaperones the SNARE complex assembly and controls neurotransmitter release. α-Synuclein can rescue neurodegeneration in CSPαKO mice. However, whether α-synuclein aggregation alters CSPα expression and function is unknown. Here we show that α-synuclein aggregation at the synapse is associated with a decrease in synaptic CSPα and a reduction in the complexes that CSPα forms with HSC70 and STGa. We further show that viral delivery of CSPα rescues in vitro the impaired vesicle recycling in PC12 cells with α-synuclein aggregates and in vivo reduces synaptic α-synuclein aggregates increasing monomeric α-synuclein and restoring normal dopamine release in 1-120hαSyn mice. These novel findings reveal a mechanism by which α-synuclein aggregation alters CSPα at the synapse, and show that CSPα rescues α-synuclein aggregation-related phenotype in 1-120hαSyn mice similar to the effect of α-synuclein in CSPαKO mice. These results implicate CSPα as a potential therapeutic target for the treatment of early-stage Parkinson's disease.
Keywords: CSPα; DNAJ chaperone family; Parkinson’s disease; synapse; α-synuclein.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Lunati A, Lesage S, Brice A.. The genetic landscape of Parkinson's disease. Rev Neurol. 2018;174(9):628–643. - PubMed
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M.. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Crowther RA, Jakes R, Spillantini MG, Goedert M.. Synthetic filaments assembled from C-terminally truncated alpha-synuclein. FEBS Lett. 1998;436:309–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

