Bridging the age gap in breast cancer: cluster randomized trial of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices
- PMID: 33760077
- PMCID: PMC10364907
- DOI: 10.1093/bjs/znab005
Bridging the age gap in breast cancer: cluster randomized trial of two decision support interventions for older women with operable breast cancer on quality of life, survival, decision quality, and treatment choices
Abstract
Background: Rates of surgery and adjuvant therapy for breast cancer vary widely between breast units. This may contribute to differences in survival. This cluster RCT evaluated the impact of decision support interventions (DESIs) for older women with breast cancer, to ascertain whether DESIs influenced quality of life, survival, decision quality, and treatment choice.
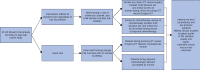
Methods: A multicentre cluster RCT compared the use of two DESIs against usual care in treatment decision-making in older women (aged at least ≥70 years) with breast cancer. Each DESI comprised an online algorithm, booklet, and brief decision aid to inform choices between surgery plus adjuvant endocrine therapy versus primary endocrine therapy, and adjuvant chemotherapy versus no chemotherapy. The primary outcome was quality of life. Secondary outcomes included decision quality measures, survival, and treatment choice.
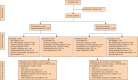
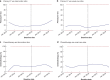
Results: A total of 46 breast units were randomized (21 intervention, 25 usual care), recruiting 1339 women (670 intervention, 669 usual care). There was no significant difference in global quality of life at 6 months after the baseline assessment on intention-to-treat analysis (difference -0.20, 95 per cent confidence interval (C.I.) -2.69 to 2.29; P = 0.900). In women offered a choice of primary endocrine therapy versus surgery plus endocrine therapy, knowledge about treatments was greater in the intervention arm (94 versus 74 per cent; P = 0.003). Treatment choice was altered, with a primary endocrine therapy rate among women with oestrogen receptor-positive disease of 21.0 per cent in the intervention versus 15.4 per cent in usual-care sites (difference 5.5 (95 per cent C.I. 1.1 to 10.0) per cent; P = 0.029). The chemotherapy rate was 10.3 per cent at intervention versus 14.8 per cent at usual-care sites (difference -4.5 (C.I. -8.0 to 0) per cent; P = 0.013). Survival was similar in both arms.
Conclusion: The use of DESIs in older women increases knowledge of breast cancer treatment options, facilitates shared decision-making, and alters treatment selection. Trial registration numbers: EudraCT 2015-004220-61 (https://eudract.ema.europa.eu/), ISRCTN46099296 (http://www.controlled-trials.com).
© The Author(s) 2021. Published by Oxford University Press on behalf of BJS Society Ltd.
Figures

References
-
- Lavelle K, Moran A, Howell A, Bundred N, Campbell M, Todd C.. Older women with operable breast cancer are less likely to have surgery. Br J Surg 2007;94:1209–1215 - PubMed
-
- Jauhari Y, Gannon M, Medina J, Cromwell D, Horgan K, Dodwell D.. National Audit of Breast Cancer in Older Patients Annual Report. Healthcare Quality Improvement Partnership, London, UK: Royal College of Surgeons of England. 2018.
-
- Derks MGM, Bastiaannet E, Kiderlen M, Hilling DE, Boelens PG, Walsh PM. et al. Variation in treatment and survival of older patients with non-metastatic breast cancer in five European countries: a population-based cohort study from the EURECCA Breast Cancer Group. Br J Cancer 2018;119:121–129 - PMC - PubMed
-
- Morgan J, Richards P, Ward S, Francis M, Lawrence G, Collins K. et al. Case-mix analysis and variation in rates of non-surgical treatment of older women with operable breast cancer. Br J Surg 2015;102:1056–1063 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

