Endoplasmic reticulum stress/XBP1 promotes airway mucin secretion under the influence of neutrophil elastase
- PMID: 33760106
- PMCID: PMC7979262
- DOI: 10.3892/ijmm.2021.4914
Endoplasmic reticulum stress/XBP1 promotes airway mucin secretion under the influence of neutrophil elastase
Abstract
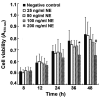
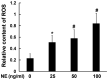
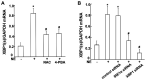
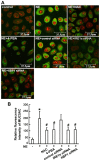
Endoplasmic reticulum (ER) stress is an important reaction of airway epithelial cells in response to various stimuli, and may also be involved in the mucin secretion process. In the present study, the effect of ER stress on neutrophil elastase (NE)‑induced mucin (MUC)5AC production in human airway epithelial cells was explored. 16HBE14o‑airway epithelial cells were cultured and pre‑treated with the reactive oxygen species (ROS) inhibitor, N‑acetylcysteine (NAC), or the ER stress chemical inhibitor, 4‑phenylbutyric acid (4‑PBA), or the cells were transfected with inositol‑requiring kinase 1α (IRE1α) small interfering RNA (siRNA) or X‑box‑binding protein 1 (XBP1) siRNA, respectively, and subsequently incubated with NE. The results obtained revealed that NE increased ROS production in the 16HBE14o‑cells, with marked increases in the levels of ER stress‑associated proteins, such as glucose‑regulated protein 78 (GRP78), activating transcription factor 6 (ATF6), phosphorylated protein kinase R‑like endoplasmic reticulum kinase (pPERK) and phosphorylated (p)IRE1α. The protein and mRNA levels of spliced XBP1 were also increased, and the level of MUC5AC protein was notably increased. The ROS scavenger NAC and ER stress inhibitor 4‑PBA were found to reduce ER stress‑associated protein expression and MUC5AC production and secretion. Further analyses revealed that MUC5AC secretion was also attenuated by IRE1α and XBP1 siRNAs, accompanied by a decreased mRNA expression of spliced XBP1. Taken together, these results demonstrate that NE induces ER stress by promoting ROS production in 16HBE14o‑airway epithelial cells, leading to increases in MUC5AC protein production and secretion via the IRE1α and XBP1 signaling pathways.
Keywords: endoplasmic reticulum stress; mucin 5AC; reactive oxygen species; inositol‑requiring kinase 1α; X‑box‑binding protein 1; respiratory inflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

