Cytokine secretion and pyroptosis of cholesteatoma keratinocytes mediated by AIM2 inflammasomes in response to cytoplasmic DNA
- PMID: 33760111
- PMCID: PMC7974272
- DOI: 10.3892/mmr.2021.11983
Cytokine secretion and pyroptosis of cholesteatoma keratinocytes mediated by AIM2 inflammasomes in response to cytoplasmic DNA
Abstract
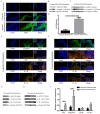
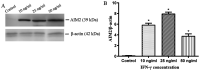
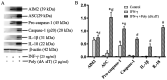
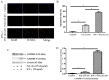
Cholesteatoma constitutes an acquired benign epidermal non‑permanent bone lesion that is locally destructive and patients often relapse. Inflammasomes, which mediate the maturation and production of IL‑18 and IL‑1β, resulting in pyroptosis, have been documented to serve a core function in multiple inflammatory conditions. Absent in melanoma 2 (AIM2) is an inflammasome that identifies cytoplasmic DNA and has previously been reported as a pivotal modulator of inflammatory responses. Therefore, the present study aimed to determine the expression levels of AIM2 in human cholesteatoma tissues, and elucidate its function in modulating cytokine production. The expression levels of IL‑18, apoptosis‑associated speck‑like protein containing a CARD (ASC), IL‑1β, AIM2 and caspase‑1 were markedly elevated in cholesteatoma tissues. Protein expression levels of AIM2, caspase‑1 and ASC were localized in the cellular cytoplasm, primarily in the granular and prickle‑cell layers in the cholesteatoma epithelium. Induction using IFN‑γ, as well as cytoplasmic DNA markedly activated the AIM2 inflammasome and elevated the release of IL‑18 and IL‑1β in human cholesteatoma keratinocytes. IFN‑γ was found to enhance poly(dA:dT)‑induced pyroptosis of cells and cytokine production. The results of the present study revealed that AIM2 expressed in human cholesteatoma serves a vital function in the inflammatory response by initiating the inflammasome signaling cascade in cholesteatoma.
Keywords: cholesteatoma; absent in melanoma 2; inflammasome; cytoplasmic DNA; cytokine; pyroptosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis.Front Immunol. 2018 Jun 4;9:1197. doi: 10.3389/fimmu.2018.01197. eCollection 2018. Front Immunol. 2018. PMID: 29915579 Free PMC article.
-
Quercetin inhibits the poly(dA:dT)-induced secretion of IL-18 via down-regulation of the expressions of AIM2 and pro-caspase-1 by inhibiting the JAK2/STAT1 pathway in IFN-γ-primed human keratinocytes.Biochem Biophys Res Commun. 2018 Sep 3;503(1):116-122. doi: 10.1016/j.bbrc.2018.05.191. Epub 2018 Jun 6. Biochem Biophys Res Commun. 2018. PMID: 29857000
-
Knockdown of the AIM2 molecule attenuates ischemia-reperfusion-induced spinal neuronal pyroptosis by inhibiting AIM2 inflammasome activation and subsequent release of cleaved caspase-1 and IL-1β.Neuropharmacology. 2019 Dec 1;160:107661. doi: 10.1016/j.neuropharm.2019.05.038. Epub 2019 Jun 8. Neuropharmacology. 2019. PMID: 31181224
-
Role of AIM2 inflammasome in inflammatory diseases, cancer and infection.Eur J Immunol. 2019 Nov;49(11):1998-2011. doi: 10.1002/eji.201848070. Epub 2019 Aug 14. Eur J Immunol. 2019. PMID: 31372985 Free PMC article. Review.
-
Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases.J Cell Physiol. 2020 Apr;235(4):3207-3221. doi: 10.1002/jcp.29268. Epub 2019 Oct 17. J Cell Physiol. 2020. PMID: 31621910 Review.
Cited by
-
Dihydrotanshinone I Specifically Inhibits NLRP3 Inflammasome Activation and Protects Against Septic Shock In Vivo.Front Pharmacol. 2021 Oct 14;12:750815. doi: 10.3389/fphar.2021.750815. eCollection 2021. Front Pharmacol. 2021. PMID: 34721038 Free PMC article.
-
Expression of periostin in the epithelium of cholesteatoma with different degrees of ossicular chain destruction and its clinical value in predicting postoperative hearing recovery.Biomed Eng Online. 2024 Nov 29;23(1):125. doi: 10.1186/s12938-024-01319-8. Biomed Eng Online. 2024. PMID: 39614252 Free PMC article.
-
Review of potential medical treatments for middle ear cholesteatoma.Cell Commun Signal. 2022 Sep 19;20(1):148. doi: 10.1186/s12964-022-00953-w. Cell Commun Signal. 2022. PMID: 36123729 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

